Project methodology
A low electricity conducting liquid model is developed in a small scale Hall-Heroult cell model to determine the bubble creation, coalition and motion in a horizontal negatively charged electrode surface and the character of electricity between this and positively charged electrodes when a magnetic field is introduced. The chemical compositions of the materials used are CuSO4 and H2SO4 respectively. When balancing with the Hall-Heroult cell, copper is resided at the positively charged electrode and bubbles of oxygen are produced under the negatively charged electrode. This whole procedure of bubble creation, coalition and disengagement are observed under very effective high speed cameras.
Apparatus used
Developing the electrolyte cell
This cell of quarter scale in relation to the actual is developed by conserving the numerical and dynamic similarities of the actual Hall-Heroult with the fiber material made from glass. There are five dimensions of the numerical values to be considered as follows.
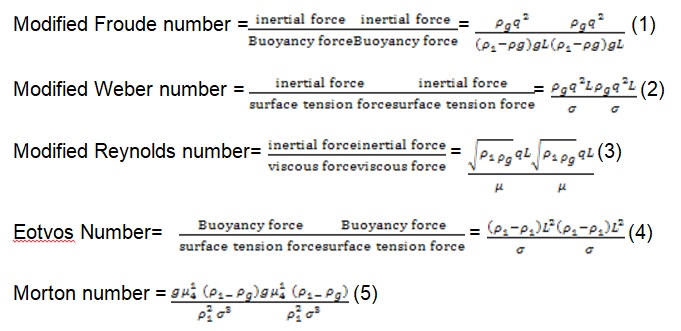
From equation (1, 2, 3, 4, 5), where q is gas generation rate per unit surface area, g is acceleration due to gravity ρ1ρ1 is the liquid density and ρgρg is density of the gas. σ is surface tension of the surrounding liquid; L is the characteristic dimension and μ1μ1 is the viscosity of the surrounding liquid. From this it is found that Buoyancy force and inertial forces developed by the evolving gas are driving forces for the movement of the bubbles and bath motion. The Eotvos number and Morton number together are used to characterize the size of the bubble and shape moving in the surrounding liquid. Eotvos and Morton number are considered for the dynamic analysis.
Electrolyte
Chemical and Physical features
The electrolyte used in this case is made up of copper Sulphate since this substance is stable enough both when in storage and when being used.
Conductors
Different conductors also known as electrodes are involved in studying the characteristics of negatively charged electrodes. The conductors used in this experiment include:
- Carbon – carbon
- Carbon – stainless steel
- Carbon- Copper
- Copper- copper
- Copper- stainless steel
- Carbon- stainless steel
These however cannot be used as both the negatively and positively charged electrodes and this phenomenon is explicated in the following analysis.
Source of Power
The electrolysis process is conducted with a power consumption of around 30A of electricity. The positive link originating from a positive terminus is directed to the positively charged electrode while the negative link is directed to the negatively charged electrode. Constant power supply has to be maintained and the consumption recorded regularly for monitoring purposes.
Magnets
The magnets used have to be strong with pull strength of up to 294kgs to enable the study of character of the current going through the negatively and positively charged electrodes when magnetic fields are present to determine whether they affect the conduct of electricity.
Camera
The cameras used must have high capture speed required to develop accurate graphs in the bubble creation process. This enables precise computation of the bubble size and the angle in which it establishes contact with the positively charged electrode surface.
Methodology
The set up developed for experimental purposes is usually a dimension of 250mm by 100mm by 150mm. The measurements of both electrodes are 150mm by 20mm and 125mm by 20mm for the anode and cathode respectively, arrived at by using the quarter scale of measurements in the actual cell. The separation between the cathode and anode remains 50mm while the latter is positioned at 110mm high so that the bubble development process can be monitored and captured accurately. The cathode on the other hand is positioned in the lower part of the cell. At this point, the electrodes are plugged in to the source of power and electrolysis takes place, as in the chemical equation below:
CuSO4(aq) +2e- +H2O = Cu(s)+ H2SO4(aq)+1/2O2(g)
From this, it is deduced that the electrically charged copper and hydrogen move towards the positive electrode. The electrically charged particles of copper are used up by obtaining negatrons from positively charged electrodes and the copper sediments remain at the positively charged electrodes.
Chemical reaction
Cu (++) + 2e (-) = Cu
Anode
The Sulphate and hydroxide sub atomic particles tend to move towards negative ions where the later surrender their electrons hence forming a radical structure. Sulphate however cannot survive in nonaligned electrical status and it therefore sets on the copper anode, the end result being copper Sulphate.
Chemical reaction
SO4 (- -) = SO4+2e-
Cathode
The size and diameter of the bubbles separated from anode plane is determined by the cameras operating at a high speed of 200 frames per second in diverse positions.