Introduction
The specific macromolecular structure of polymers remains a challenge to study because of the complexity and potential heterogeneity of molecular distribution. In turn, the demand for these data is high because of the direct influence of polymer structure on its physicochemical properties. In this context, the study of the linear, water-soluble polymer polyvinyl acetate alcohol (PVOH) synthesized from vinyl alcohol monomers is of high interest. In normal synthesis, the polymer exhibits a weakly branched and ordered structure, whereas the use of methanolysis of polyvinyl acetate (PVAc) yields a configuration with a reversible monomer arrangement, as shown in Figure 1 (Shoemaker et al., 2018).
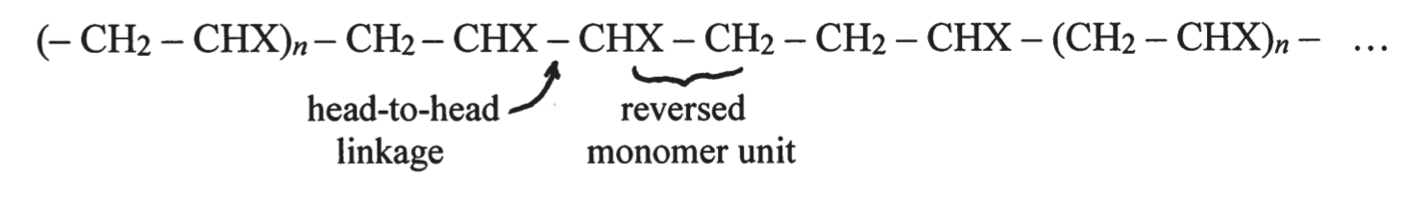
The ratio of frequencies of direct and reversible monomers is determined by the formula:
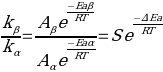
In this formula, S corresponds to the steric coefficient, which determines the probability that straight and reversible monomers will form bonds and no obstacles will be created for this. The present experiment takes advantage of the fact that the formation of a direct monomer in the chain, actually 1,2-glycol, can be detected by periodate action, using the Flory and Leutner method (Kanda et al., 2020). Thus, by measuring the molar mass of the PVOH samples before and after treatment, it becomes possible to statistically determine the macromolecular distribution. This is done using the intrinsic viscosity of the polymer and the time it takes for it to pass through a vertical capillary. In particular, treatment with periodate is expected to break the macromolecular chain and produce smaller fragments whose molecular weight is lower than that of the untreated solution.
Experiment/Procedure
This experiment was performed in strict accordance with Experiments in Physical Chemistry (7th ed.) by Garland, Nibler & Shoemaker unless otherwise noted (Shoemaker et al., 2018). For this experiment, a pre-prepared PVOH polymer solution was used, or, if not available, a working solution was prepared according to the methodological instructions. Six solutions were prepared, two of each concentration: in each pair, one and the solutions were subjected to cleavage by the action of periodate, and the other was not. A vertical capillary and a timer were then used to measure the time each of the solutions flowed out of the vessel at 25°C. In total, considering the control measurement for water, seven lines of experiments were performed with three attempts in each.
Conclusion
The purpose of the present experiment was to study the phenomenon of the reversible ordering of monomers in the polyvinyl acetate alcohol polymer. During the synthesis of PVOH by methanolysis, some parts of the macromolecular chain have molecules arranged not “head-to-tail” as is normal but “head-to-tail.” As a consequence, chemolysis of such polymers with periodate allows obtaining sections of the original chain of different lengths, which directly affects the viscosity of the product (Hershberger, 2020). It was viscosity as an indicator of polymer chain breakage that was used for quantitative analysis in this experiment. It was shown that the higher the concentration of the polymer in the solution, the slower it flows through the vertical capillary. In addition, the viscosity and specific viscosity of the solution at each polymer concentration were calculated, and then the characteristic viscosity was plotted when the concentration of the substance was theoretically zero or at the limit of dilution.
It was found that the characteristic viscosity of the undiluted solution — unaffected by the periodate — was 3.67 times higher. In addition, this value was calculated by means of additional graphs, and the difference between the two results did not exceed 3%, which indicates a high degree of accuracy of the measurement. Thus, a higher value of the solution outflow time expressed in a higher value of the characteristic viscosity is reliable evidence of an increase in the molecular weight of linear macromolecules. Hence, it was proved that with periodate cleavage, it is indeed possible not only to break PVOH molecules but also to determine the quality of this chain breakage. In addition, it was demonstrated that by measuring the flow time of the solution through the capillary, it is possible to determine the molecular weight of the substance in the solution with high accuracy. In this experiment, the calculated mass was 5.75 times lower for the solution that underwent chemolysis compared to the solution without treatment, which is in entire agreement with theoretical expectations. Finally, using the found molecular weight of the substance, the frequency of occurrence of abnormal monomers in the chain was calculated, which fully answered the research question of the experiment.
References
Kanda, T., Kitawaki, M., Arata, T., Matsuki, Y., & Fujiwara, T. (2020). Structural analysis of cross-linked poly (vinyl alcohol) using high-field DNP-NMR. RSC Advances, 10(14), 8039-8043.
Shoemaker, D. P., Garland, C. W., & Steinfeld, J. I. (2018). Experiments in physical chemistry. McGraw-Hill.
Hershberger, S. (2020). Intrinsic viscosity, evaluating the polymerization pattern in polyvinyl alcohol[PDF document]. Web.