Introduction
The human body is a complex array of body systems designed to harmoniously coexist and perform their designated functions in a synergic manner. The synergic relationships among the body systems lead to adverse effects on the body’s physiological processes in case one of the body systems is afflicted by a disorder of any nature. The affliction can result from a disease or a fatal accident. There are a plethora of diseases with the potential of adversely affecting the normal functioning of body systems. A prime example is a leukemia, which comes in various forms and with varying magnitudes of fatality (Mayo Clinic, 2013). This essay thus sets out to examine leukemia with a focus on its etiology, pathogenesis, morphological changes, clinical significance, and basic management in a bid to develop a clear concept of all aspects of the disease.
Overview of Leukemia
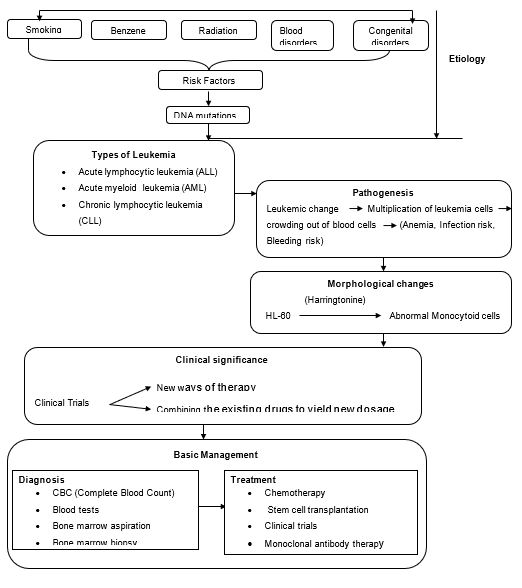
Leukemia is a form of blood cancer, which manifests in four distinctive categories viz. “acute lymphoblastic (lymphocytic) leukemia (ALL), acute myeloid (myelogenous), leukemia (AML), chronic lymphocytic leukemia (CLL), and chronic myeloid (myelogenous) leukemia (CML)” (American Cancer Society, 2012, p.4). The disease has been reported to affect cats, guinea pigs, and cattle as well (The Leukemia & Lymphoma Society, 2012). Studies are ongoing to establish the exact cause of the disease, which is still unknown according to the Leukemia and Lymphoma Society (2012). Similarly, the disease currently has no known cure, but it has an array of treatment approaches, which focus on remission (American Cancer Society, 2012).
Causes of Leukemia
Research has increasingly linked the disease to some known substances or occurrences, which are considered as its risk factors. These factors include “smoking, exposure to certain chemicals such as benzene, exposure to high-dose radiation, certain blood disorders such as myeloproliferative disorders, and congenital disorders such as Down syndrome among many others” (American Cancer Society, 2012, p.8). However, researchers have not yet successfully articulated these risk factors to leukemia, but they have established some insightful developments that occur in the bloodstream, which could lead to leukemia. The American Cancer Society (2012) posits that during cell division, the mother cell makes a new copy of DNA in its chromosomes. Such DNA changes are the principal causes of cancers of which leukemia is part. The changes that might occur include “translocations, deletions, inversions, and addition” (American Cancer Society, 2012, p.12). How exactly these changes lead to leukemia is still unknown, but it is generally believed that these changes could cause leukemia by virtue of the fact it is cancer.
Pathogenesis of Leukemia
As noted earlier, the exact cause of leukemia is still unknown. However, what is clear so far is that once the marrow cell undergoes a leukemic change, leukemia cells thrive and eventually crowd out normal cells (The Leukemia & Lymphoma Society, 2012). The rate at which this growth happens varies notably among the various types of leukemia. In the case of ALL and AML, after the leukemic change occurs, one leukemia cell multiplies into a trillion or more similar cells thus crowding out normal cells both in the blood and in the marrow (The Leukemia & Lymphoma Society, 2012). This scenario leads to deficiency in “red blood cells (anemia), white blood cells (infection risk) and platelets (bleeding risk)” (The Leukemia & Lymphoma Society, 2012, p.19).
On the other hand, chronic myeloid leukemia takes a different approach in its proliferation. The cell that perpetuates it continues to produce the red and white blood cells together with platelets almost normally, but with a reduction in the count of red blood cells causing anemia. The white blood cells are produced in larger numbers than normal such that if unchecked, the white blood cells could crowd out red blood cells thus worsening the state of anemia and slowing the flow of blood (Patlak, 2001).
Chronic lymphocytic leukemia, on the other hand, propagates itself by producing numerous nonfunctional lymphocytes. “The non-functional cells crowd out normal cells in both marrow and the lymph nodes” (Mayo Clinic, 2013, Para.5). By so doing, the leukemia cells interfere with the normal functions of lymphocytes thus weakening a patient’s immunity (The Leukemia & Lymphoma Society, 2012). Crowding out normal blood-forming cells from the marrow also leads to deficiency in blood cells thus causing anemia, vulnerability to infection, and risk of hemorrhage (The Leukemia & Lymphoma Society, 2012).
Morphological Changes of Leukemia
Leukemia cells, HL-60, when exposed to antileukemic drug Harringtonine, exhibit some morphological changes resulting in “abnormal monocytoid cells whose appearance paralleled the decrease in circulating blast cells” (Patlak, 2001, p.21). This was established by a study that was conducted based on the premise that Harringtonine acts by preventing the formation of protein (Patlak, 2001). The study thus adopted two different approaches in a bid to verify this premise.
To begin with, a detailed analysis of Harringtonine’s vitro effect on cloned HL-60 promyelocytic leukemia revealed that leukemia cells when treated with Harringtonine, were triggered to transform into monocytic cells and that this was paralleled by a notable impediment of the proliferative prospective of the cells (Patlak, 2001). Similarly, the second approach, which involved analyzing the effects of the drug on freshly isolated leukemia cell populations, yielded the same results (Patlak, 2001). This similarity in the outcome of the two tests points to the fact that the drug achieves its goal by causing morphological changes, which transform leukemia cells into forms whose proliferative capabilities are less fatal, thus reducing its ability to adversely affect the body.
Clinical Significance
Leukemia’s clinical significance in contemporary times lies in the fact that it has spurred numerous research studies, which analysts tout as being likely to produce a cure for leukemia in the near future. Such studies lead to the conduction of clinical trials, which at times yield new ways of carrying out therapy or new ways of combining the existing drugs to come up with new dosages. The clinical trials often target newly diagnosed leukemia patients, those who do not get a good response to treatment, patients who relapse after treatment, and those who continue treatment after remission (American Cancer Society, 2012). Being close to making a breakthrough normally has a tendency of fuelling more activity in the same direction to hasten the breakthrough, and this aspect underscores the extent of leukemia’s clinical significance.
Basic Management
The disease, as noted earlier, is most common among people above 60 years of age. Its prevalence is also notable among children. The most common types of leukemia among adults include “AML and CLL while children mostly suffer from ALL” (American Cancer Society, 2012, p.36). The general symptoms of leukemia include “tiredness or no energy, shortness of breath during physical activity, pale skin, mild fever or night sweats, slow healing of cuts and excess bleeding, black-and-blue marks (bruises) for no clear reason, and aches in bones or joints (for example, knees, hips or shoulders) to name a few” (American Cancer Society, 2012, p.36).
Diagnosis of leukemia is done via a complete blood count (CBC). This test gives statistics on the blood cells and helps establish if any blood cells are low in supply. In a bid to determine the type of leukemia, CBC is coupled with several other tests such as blood tests, bone marrow aspiration, and bone marrow biopsy (American Cancer Society, 2012). Once diagnosed, treatment follows in the form of chemotherapy, stem cell transplantation, or clinical trials in cases of acute leukemia (American Cancer Society, 2012). For chronic myeloid leukemia, there is a list of three newly approved drugs for diagnosed patients to choose from and they include “imatinib mesylate, dasatinib or nilotinib)” (Quintás-Cardamaemail & Jabbour, 2013, p.487). In the case of chronic lymphocytic leukemia, there are patients who may not need medication for a long time after diagnosis. However, if treatment becomes necessary, it takes the form of chemotherapy or monoclonal antibody therapy alone or in combination (American Cancer Society, 2012).
Conclusion
Leukemia, as outlined by the essay, is a multifaceted disease, which comes with varying levels of severity. However, the type and level of severity notwithstanding, the disease needs to give due attention so that its cure might be found. Such a breakthrough will present its casualties with great relief from its dehumanizing symptoms and effects. Clinical trials that look promising are underway and hopefully, a cure for the disease will be discovered to tame this cancer.
References
American Cancer Society: Leukemia–Acute Myeloid (Myelogenous). (2012). Web.
Mayo Clinic: Leukemia. (2013). Web.
Patlak, M. (2001). Targeting Leukemia: From Bench to Bedside. Web.
Quintás-Cardamaemail, A., & Jabbour, E. (2013). Considerations for early switch to nilotinib or dasatinib in patients with chronic myeloid leukemia with inadequate response to first-line imatinib. Leukemia Research, 37(5), 487-495.
The Leukemia & Lymphoma Society: Understanding Leukemia. (2012). Web.
Concept Map for Leukemia