This review discusses the fourth phase of a comprehensive project focused on the development of an efficient adsorption storage facility for natural gas aimed at reducing daily peak load. Among the essential requirements of future storage design are the criteria of mechanical strength, high material adsorption capacity, chemical stability, and scalability to the industrial market. It should also be recalled that the previous phase of the comprehensive project was completed by finding a material that was optimal from the production and technical point of view but not suitable from the economic point of view. That was Maxsorb granular activated carbon, which, despite all its advantages, proved to be extremely expensive for permanent use. More precisely, the cost per kilogram of this material exceeded the established range by more than thirteen times, so Maxsorb granular activated carbon was not an utterly suitable solution for the storage project. As a consequence, there remains a need to search for alternatives that, combined with excellent physical and chemical characteristics, would have suitable economic benefits to start production. This report examines ten different types of activated carbon, which use the volumetric method to measure adsorption capacity. The storage capacity is used as a quantitative measure of adsorption capacity.
Table 1. Information on the ten types of activated carbon used to study their adsorption and economic characteristics.
The table above provides background information on the ten types of activated carbon studied, indicating their place of manufacture, shape, and name on the label, which will be used in the following discussion. Notably, for five of the ten samples at room temperature and 65 bar pressure, the storage capacity is over 120, which is potentially interesting for this study. These five materials are listed below in order of decreasing storage capacity:
- YL-1
- YL-2
- SRD08080
- SRD08017
- SZD
Methodology for Volumetric Measurement of Solid Adsorption
For solid-state materials used in the chemical industry, the absorption capacity must be strictly defined. One of the traditional methodologies for this determination is the volumetric method, whereby an isolated vessel containing the solid substance to be analyzed is treated with a sorbent gas. The change in pressure in the apparatus initiates adsorption of the gas in the volume of the solid substance, with the amount of absorbed gas being the difference from the gas remaining in the vessel, indicating the adsorption capacity of the gas. The accuracy of determining the absorbed gas is determined by the material balance equation if the sum of the volumes of the internal voids of the solid material is at least approximately known.
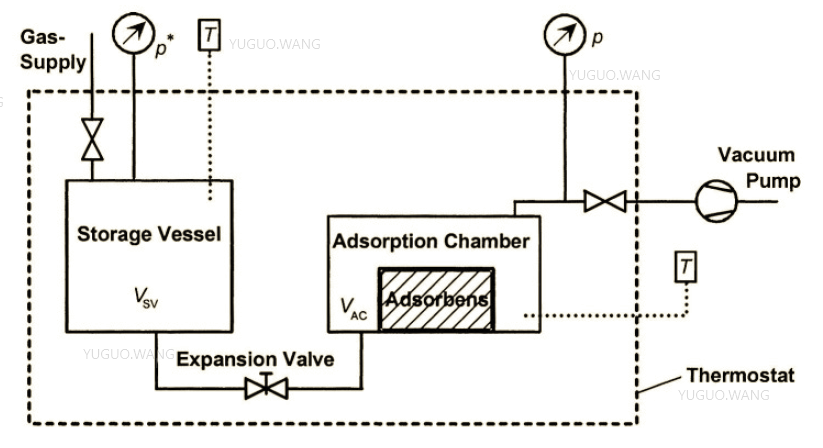
A typical setup for volumetric measurements consists of a gas storage chamber Vsv and an adsorption chamber Vac. Communications are provided by connecting tubes and valves, with both chambers placed in a thermostat connected to temperature and pressure sensors, as shown in Figure 1. When the expansion valve is opened, gas from chamber Vsv of mass m* enters the second chamber, in which adsorption on activated carbon occurs. Reaching the thermodynamic equilibrium state, at which the pressure and temperature inside the vessels are constant, requires time, and the duration of the process can vary from milliseconds to several days. Mathematically, this process can be written as:
where ma is the mass of adsorbed gas as a result of adsorption and mf is the sorbed gas at the moment of equilibrium. It is noteworthy that mf can also be determined from the EOS as:
where M is the molar mass for the sorbed gas, R is the universal gas constant, T is the temperature, and Z(p,T) is the compressibility coefficient depending on the real temperature and pressure inside the chambers. This equation also includes Vf as the volume of the sorbed gas, which defines as:
where VHe is the empty volume of the sorbate phase and sorbent material protected from gas penetration inside. Thus, through the series of equations described, it becomes possible to determine the mass of absorbed gas ma.
Characteristics of the Materials Used
Ten potentially interesting samples of activated carbon were selected for this study, which can be used for industrial purposes afterward. Experimental characteristics, including bulk and skeletal densities and the mass of the material used, are shown in Table 2.
Table 2. Data on densities and amounts of charcoal used for the ten species.
Experimental Procedure
Volumetric methodology for determining the absorptivity of activated carbon was used in this experiment to obtain data for ten sample materials. Methane was used as the absorbent gas, as required by the objective of the comprehensive project. The adsorber chamber had a volume of 124 cm3, while the volume of the tube to be connected to the chamber was 55 cm3. A vacuum pump for degassing was also connected to the unit, and the adsorber was covered with a heat jacket, allowing the desired temperature of the adsorber to be set for measurement. The general view of the entire used construction is shown in Figure 2.
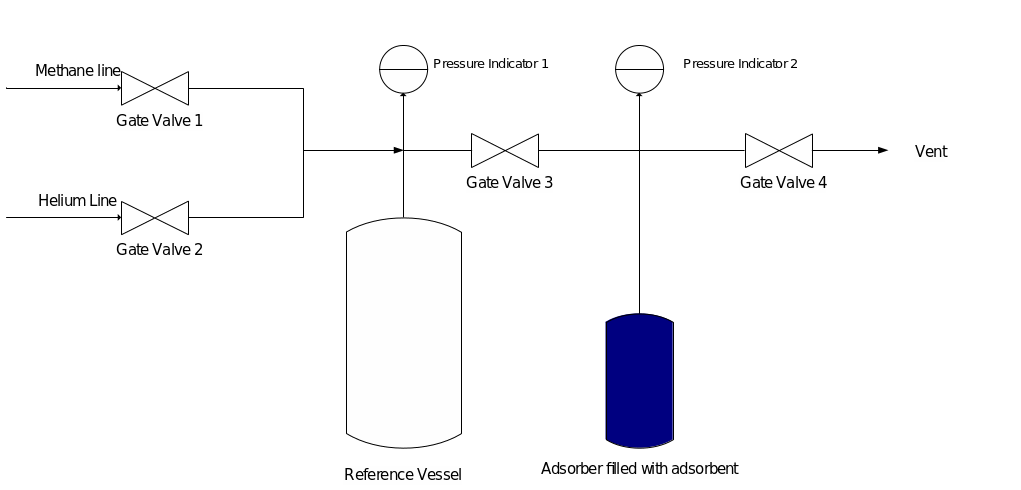
The adsorber was filled with 50 grams of granular activated carbon, which was degassed at 120°C for and 2.5•10-4 torr pressure for 4 hours. Using a heat jacket, the adsorber was cooled to five different temperatures, including 10, 15, 25, 30, and 35 °C. Strictly two liters of methane were pumped into the gas chamber and adsorbed on the coal when the expansion valve was opened. Data were collected and recorded using SCADA software.
Results
Three kinds of independent plots were plotted for each of the activated charcoal species, including a D-A isotherm plot, a D-A adsorption equation plot, and a volume capacity plot. The parameters shown in Table 3 reflect the results of W0 and E0 measurements at room temperature and 65 bar.
Table 3. Measurements of W0 and E0 Values for Each Activated Carbon Sample.
Comparison of the Models
For each of the ten samples, volumetric capacity plots were plotted at a set temperature of 25 degrees Celsius and a pressure of 65 bar. Figure 3 shows a comparison of the curves.
It is noteworthy that out of the ten curves, only five had the best absorption capacity characteristics. A separate graph was plotted for them (Figure 4), comparing these five graphs in more detail.
Conclusion
The overall conclusion of the entire experiment shows that only five of the ten activated carbon samples had excellent absorptivity characteristics. In particular, at a pressure above 50 bar and a temperature of 25 degrees Celsius, the capacity could be higher than 120. Thus, the following five samples showed the best results in the ranking from the highest to the lowest absorption capacity.
- YL-1
- YL-2
- SRD08080
- SRD08017
- SZD