Abstract
The 2009 H1N1 influenza pandemic started in Mexico last spring and recently declared a pandemic by the WHO has brought the 1918 pandemic to memories with its cost of millions of lives. Efforts to acquire more knowledge about the virus, develop treatments, produce vaccines, and come up with better prevention and prophylaxis strategies are escalating all over the world. Therefore, there is a need to provide an overlook or a synopsis of the current knowledge about this pandemic. This essay aims to provide a synopsis of H1N1 infection in light of the recent outbreak.
Introduction
There are three types of influenza viruses, classified according to infection severity. First, type A that causes the severest form of infection, type B and type C cause less severe forms of infections with type C being the weakest. Another classification of influenza viruses is according to the chemical structure of surface proteins (antigens) of the virus particle. There are two major proteins; hemagglutinin (known as H antigen) and neuraminidase (known as N antigen), both proteins are essential for virus replication and survival. More than 10 forms of H antigen and 9 identified N antigens are known; thus, the influenza viruses are identified as H1N1, H5N1, and H3N2…etc. Neuraminidase (N protein) as the name points is an enzyme that facilitates the new viruses escape from the cell once replicated. It breaks the sugar-protein linkage at the surface of the infected cell (Potter, 2000, pp. 254, 255). The H1N1 virus is an influenza A virus subtype that commonly infects pigs, which gave it the common name swine flu. The virus crossed the species barriers sporadically to infect humans, yet cases of human-human transmission were occasional until March-April 2009 when an outbreak occurred in Mexico (Myers et al 2007).
The virus
Brief historical background
Influenza A viruses are omnipresent, the H1N1 subtype infects humans, pigs, and birds. Epidemiologists believe it was responsible for the 1918 influenza pandemic (worldwide epidemic) when over 20 million people died because of the disease. Despite that, the first isolation of the H1N1 virus from a human was in 1974 (Smith et al 1976). In 1976, a local epidemic of swine flu virus with severe respiratory illness occurred in Fort Dix (New Jersey) affecting 13 soldiers with one death. In this epidemic, there was no history of exposure to pigs for any of the cases (Gaydos et al 1977). There is evidence suggesting that pigs are not only reservoir hosts to H1N1, but also act as mixing bags for the genetic assortment of the virus because they are susceptible to infection from birds, cats, and other mammals. Isolation of H1N2 viruses in Japan and the variant H3N2 in Italy from pigs proves that genetic remixing between influenza viruses occurs naturally in pigs with the possibility of producing new human pandemic strain (s) (Wentworth et al 1994).
Evolutionary changes and emergence of the 2009 pandemic H1N1 virus
The H1N1 virus responsible for the pandemic of 1918 is believed to have emerged concurrently from birds to swine and humans. Swine-origin influenza virus (S-OIV) believed to blame for the current pandemic probably emerged from swine into humans. The direct genetic event that led to the appearance of the new virus is a reassortment (genetic mix into new combinations) between two H1N1 swine viruses, which originally were the result of four unimpeded avians to mammalian cross-species transmissions. This was preceded by a minimum of four other previous reassortments of gene segments among avian, human, and swine H1N1 viruses. Because of this tangled history, S-OIV shares three genetic segments with H1N1 and three other genetic segments with H3N2 viruses. Despite repeated reassortments, it is not known if cross-immunity against remote shared H1N1 antigenic determinants (epitopes) produces clinical protection. A look back to previous epidemics shows the virus is rarely transmitted from an individual to another. The Fort Dix local epidemic was the only exception as there was no history of exposure to swine and the virus was never transmitted beyond the locale. The appearance of a triple genetically mixed (reassortants) H1N1 in 1998 in the USA heralds the tendency to override the species barrier and cause swine-human infections (Zimmer and Bruke 2009).
The H1N1 virus is an RNA virus having eight segmented genomic (nucleic acids sequence) ribonucleic acids, besides three polymerase genes (control the polymerase enzyme activity responsible for the catalysis of nucleic acids polymerization into a strand). The 8 segmented genomic RNA(s), PB2 and PA (polymerase genes) evolved from avian virus introduced to swine population in 1998, while the remaining PB1 (polymerase gene) evolved from an avian virus entered humans in 1968 (Shen and Shih 2009). Nelson et al (2008, p. 1) examined data of 71 complete genome sequences (1918-2006) to demonstrate the role played by segmental reassortment in H1N1genomic evolution. They inferred intrasubtype reassortment of H1N1 is more significant to epidemiology than previously believed.
Pathogenicity of the H1N1 virus
Multiple factors determine the pathogenicity of the H1N1 virus, however, the hemagglutinin proteins remain the most important determinant. This protein facilitates virus-host cells binding and consequent fusion of the viral and endosomal membranes for viral ribonucleic acid precursors release into the cytoplasm. Different HN subtypes have variable host cells-receptors binding capacity, thus, receptors distribution on the host cells and the virus receptor-binding capacity would explain influenza virus-host specificity. Hemagglutinin split (cleavage) is necessary for viral infectivity because it exposes an amino acid boundary (fusion peptide) that facilitates fusion of the viral envelop to the host cell endosomal membrane. PB2 protein contributes to viral pathogenicity possibly by affecting viral growth; further, both H and PB2 proteins are essential to droplet transmission. PB1 protein induces host cell apoptosis by interacting with two mitochondrial proteins, and through the same mechanism, it enhances the frequency and severity of superimposed bacterial infections (Neumann et al 2009).
Diagnosis
The first diagnostic difficulty met by the clinical staff is to answer the question is the case a seasonal flu case or a swine flu case? This needs a high suspicion index enough not to miss cases and at the same time not to create panic or lab overload. Clinically, Fever (100-102 degrees F) that last lasts for 3-4 days characterize H1N1 cases, in addition, headache, generalized body aches are severe with prominent fatigue and weakness that may last for 2-3 weeks. Upper respiratory tract manifestations are unlike seasonal flu (stuffy nose, sneezing, and sore throat) are not severe. However, cough and chest discomfort are evident and may be severe, complications are more severe in H1N1 infections commonly in the form of bronchitis and pneumonia (Sabharwal et al 2009).
Clinical diagnosis
The Novel Swine-Origin Influenza A (H1N1) Virus Investigation Team (2009) reviewed 399 cases (of 642) with available clinical data; the age range for confirmed cases was 3 months to 81 years. Fever was prominent in 94% of cases, cough in 92%, and sore throat in 66% of cases. Interestingly 25% of patients had diarrhea and-or vomiting. Nadkar et al suggested the virus’ incubation period (18-72 hours) is the time needed for inflammatory mediators to build-up followed by symptoms of acute onset. Besides, they suggested the overall vulnerability of the patient (immune suppressed, age, pregnancy…) plays an important role in determining the severity of the case at the time of presentation. Nadkar et al (2009, p.455) classified H1N1 cases into three categories; first, are suspected cases who are patients with fever (100-102 F, or 38 C) giving a history of contact with a confirmed case within 7 days before symptoms appeared. Second, are probable cases who are patients with febrile acute respiratory illness testing positive to influenza A but not sub-typed; and confirmed cases who tested positive for the H1N1 virus.
Laboratory diagnosis
Currently, there are three common methods for H1N1 (S-OIV) lab diagnosis namely rapid influenza diagnostic tests (RIDTs), real-time polymerase chain reaction (RT-PCR) assay, and virus culture. RIDTs are less expensive and available in many clinical laboratories; however, they have low sensitivity (40 to 69%), which decreases further as the virus level decreases. Despite that, they are useful to confirm, but not to rule out infection. Therefore, in positive cases, the result in interpretation should be, infection with influenza A is likely which could be H1N1, S-OIV, H3N2, or any other. In negative cases, the test result cannot rule out influenza virus infection. Second, is RT-PCR, which is the most sensitive test approved by the FDA, and is currently the best available means to differentiate H1N1 or S-OIV infection from seasonal influenza infection. Third, is virus culture and isolation for hemagglutination or immunofluorescence subtyping (Holmes et al, 2009), which has the disadvantage of being time-consuming since the virus culture takes nearly 7 days. Specimens (swabs) are taken using a synthetic material (nylon, plastic…) but not cotton swabs to minimize adsorption of the organism on the swab surface, and transferred immediately to the lab. If there must be some time before transferring the specimens, they should be kept at 4 C in an upright position for a maximum of 72 hours (Kaore et al 2009).
Radiological diagnosis of H1N1 infection
Lee et al (2009, p. 533) described the chest X-ray features of cases of H1N1 infection, which are bilateral patchy alveolar opacities associated with basal interstitial opacities taking linear, reticular, or nodular shadows. Ct findings are typical for viral pneumonia (peri-bronchial ground-glass opacities and air space consolidation) which may take an ill-defined or lobar consolidation configuration.
H1N1 infection and pulmonary disease
Chowell et al (2009, p. 674) examined 2155 cases of severe pneumonia complicating H1N1-S-OIV infection reported to the Mexican Ministry of Health in one month period (24-03 to 24-04-2009). Of these cases, 821 were hospitalized and there were 100 deaths, 87% of the deaths, and 71% of the cases were among the 5 to 59 years age group. This represented an age shift compared to the distribution of pneumonia among age groups in previous seasonal influenza epidemics (2005-2008), it also displayed a sharp rise in both rates compared to 17% and 32% for deaths and severe cases in seasonal epidemics. They also noticed a decreased rate among the 60 years or over age group possibly because of previous exposure to H1N1 infection, which may have caused cross-immunity. Perez-Padilla et al (2009, p. 680) examined 98 cases reported to one center (the National Institute of Respiratory Diseases in Mexico City) and reported nearly the same results. Interestingly, their results showed that 22 healthcare workers developed mild to moderate illness within seven days of initial contact with patients.
H1N1 infection in special populations
H1N1infection in the pediatric population
In many cases, the course of disease in children can be mild and self-limiting; however in the severe case and the underlying medical condition often exists. Boughton (2009) suggested asthma, sickle cell disease, and congenital heart disease are the commonest, Shah (2009) suggested, in India, diabetes, lung disease, heart disease, and AIDS is the commonest conditions.
Gastrointestinal manifestations are commoner than adults (49%) and accounted for one death in Boughton’s (2009) series. Febrile seizures are common, low oxygen saturation levels below 95% occurred in 35% of children with H1N1 infection, nearly 16% of children needed admission to the ICU, and 5% needed mechanical ventilation (Boughton 2009).
H1N1 infection during pregnancy
Infection with influenza A virus during pregnancy causes serious maternal and fetal effects, which include preterm labor, pneumonia, adult respiratory failure, and even maternal death. Most cases reported by the US Disease Control and Prevention Center (CDC) (15 April to 24 July 2009) were mild, and only 11% of patients required hospitalization. Despite that, the rate of hospitalization is higher among H1N1 infected pregnant females. It is imperative to evaluate these patients with serial vital signs monitoring including pulse oximetry. Guided judgment is essential for radiological evaluation and invasive procedures like arterial blood gases measurement. Because of the potential of rapid disease progression, close follow-up is always necessary especially within 24 hours of prescribing medications (Saleeby et al 2009).
H1N1 infection in immunocompromised patients
A patient who has received organ or stem cell transplant, on chemotherapy, or systemic steroids, or AIDS patients (immunocompromised) are at higher risk for H1N1 complications. Also, immunosuppression may limit vaccine response (Kunisaki and Janoff 2009). Sharma and Gupta (2009, p. 179) reported the CDC identified evidence of resistance to oseltamivir (antiviral medication) in two severely immunosuppressed patients. These patients continue shedding oseltamivir-resistant virus for longer periods; thus, they are infectious for a longer time, besides, transmit oseltamivir-resistant infection (Sharma and Gupta 2009).
Treatment, vaccination, prophylaxis, and prevention
Antiviral medications
The FDA approved two classes of antiviral drugs for the treatment and prevention of influenza virus infections namely M2 ion channel blockers, and neuraminidase inhibitors. M2 ion channel blockers like amantadine and rimantadine were effective in the treatment of influenza A but not influenza B infections as the virus lacks the M2 protein. However, all tested viruses isolated from H1N1 and S-OIV infections were resistant to these drugs. The second group (neuraminidase inhibitors) includes oseltamivir (Tamiflu) and zanamivir. Both drugs are effective in managing the H1N1 case but differ in bioavailability, the first is administered orally, while the second is by inhalation (CDC, 2009). The Center for Disease Control and Prevention advises using antiviral medications for hospitalized patients and high-risk groups; children below 5 years, adults 65 years or more, immunocompromised patients, or those with underlying chronic medical disease (after Dhamija et al 2009). The WHO advises using oseltamivir almost immediately in serious cases or cases rapidly deteriorating (after Dhamija et al 2009).
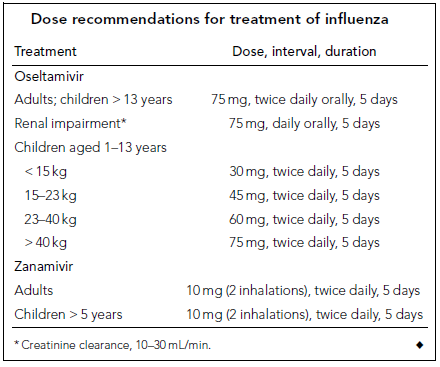
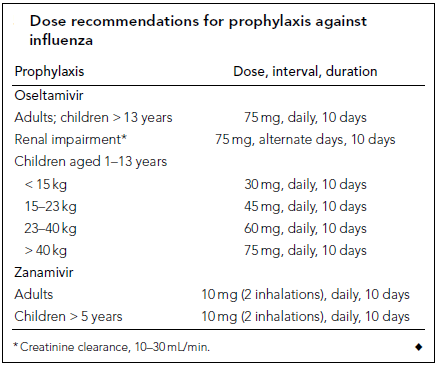
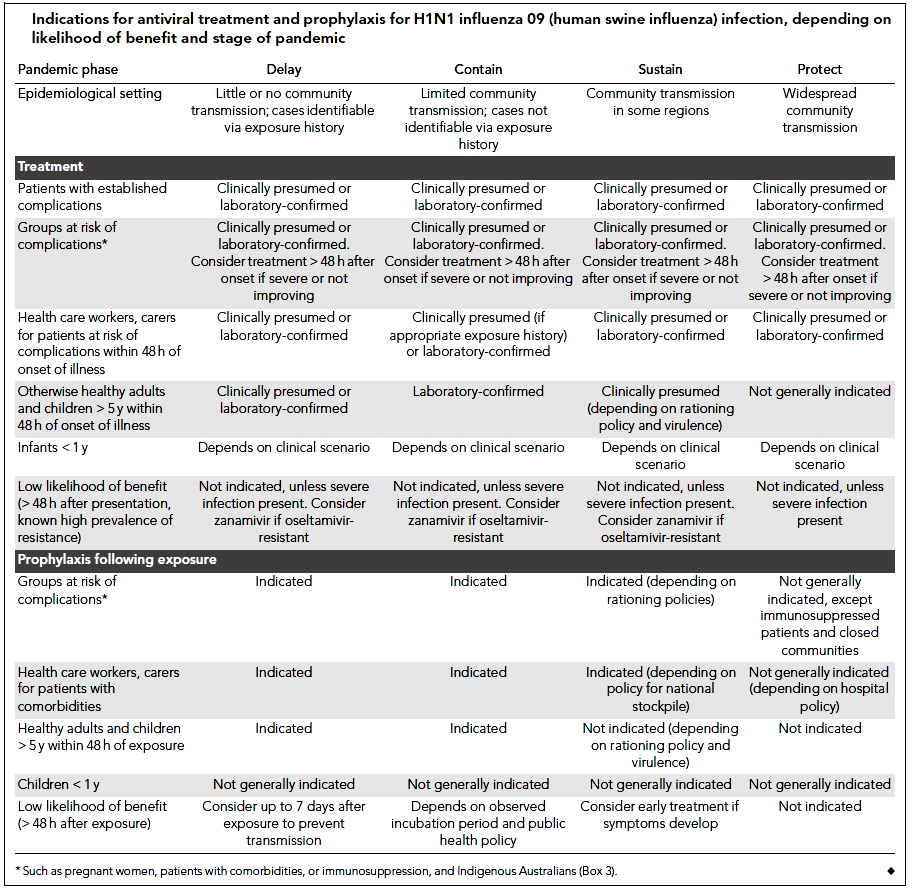
Cheng et al (2009) classified antiviral medication treatment strategies according to age, underlying medical diseases, and the case severity into four categories. 1- Patients with H1N1 09 influenza but healthy otherwise; since anti-neuraminidase agents reduce the duration of symptoms, treatment with these drugs is indicated to shorten work absence but is subjected, at all times, to public health and hospital policies. 2- In infants and children; antiviral medications are indicated in high-risk groups (5 years age or less, or with associated comorbidities), although oseltamivir showed efficacy up to 1 year age yet there is limited safety evidence below that age. However, the US FDA has approved oseltamivir use below 1 year age only in emergency authorized cases. 3- Patients at risk of complications; based on Meta-analysis, Cheng et al (2009) suggested oseltamivir reduces the incidence of lower respiratory tract infection, progression to pneumonia, and rate of hospitalization. 4. Patients with severe H1N1 09 infection; although solid evidence of efficacy in these cases is lacking; yet, based on previous experiences with H5N1 (avian flu) cases there is a clinical consensus that the drug can be used in these cases. This is based on the assumption that it may reduce viral replication which contributes significantly to the severity of the disease. Tables 1 and 2 (see appendix) show the recommended doses of anti-neuraminidase drugs for treatment and prophylaxis of H1N1 infection. Table 3 (see appendix) shows the indications for antiviral treatment and prophylaxis for H1N1 influenza (human swine influenza) infection, based on the likelihood of benefit and stage of pandemic (Cheng et al 2009).
Poland et al (2009) pleaded not to use monotherapy antiviral treatment lest resistant strains should develop; instead, they advised using combination therapy based on risks and point of care.
Vaccination
Vaccination increases population immunity, slows down the spread of infection, and decreases the epidemic height; thus, pacifies the rush to deal with epidemic cases. Despite limited uncertainties about the effectiveness of the ultimate vaccine, yet, its necessity and usefulness remain unchanged (Yang et al 2009). Trivalent influenza vaccine (H3N2, seasonal H1N1 virus, but not S-OIV, and influenza B) has been given in Mexico during the early 2009 H1N1 epidemic. The vaccine effectiveness was 73% and none of the vaccinated cases died (Garcia-Garcia et al 2009).
Greenberg et al (2009) evaluated the immunogenicity and effectiveness of the recently developed monovalent inactivated vaccine (for H1N1 2009 virus). Their results showed an antibody titer 1:40 after 21 days of vaccine administration, with no deaths or serious disease complications occurring among the 120 vaccinated individuals. Table 4 (see appendix) shows the types, dosage, and administration routes of monovalent vaccines (adapted from CDC 2009).
Prophylaxis and prevention
Using antiviral medications for chemoprophylaxis is indicated for individuals at high risk (like healthcare workers) or at high risk of acquiring complications (like those with underlying medical disease), or as an adjuvant to vaccination at the high peak point of the epidemic. Hand hygiene is probably the single most significant prophylaxis measure to reduce the risk of transmission from one individual to another. Cough hygiene is a necessity for all individuals with manifestations of respiratory infection (Dahmija et al 2009).
Quarantine and school closure
Quarantine is on two levels; contacts and international at ports. Close contacts of suspected, probable, or confirmed cases should be put to voluntary home quarantine for 7 days with monitoring of fever, notification to health authorities if symptoms develop. International quarantine at ports aims at preventing the import of novel cases or novel viruses; thus, influencing the positive control of domestic disease (Kuo et al 2009).
Sypsa and Hatzakis (2009) designed a mathematical model based on epidemiological data of the Mexico 2009 epidemic to assess school closure among other intervention strategies to affect the epidemic possible spread in Europe. They inferred a 100% school closure (in a community of 2000 population) at the threshold of 1% cumulative incidence of disease can result in an 89.3% reduction of symptomatic cases. If school closure accompanies voluntary home quarantine of contacts this may result in a reduction of incidence rate by 94.8%.
Prophylaxis and prevention among healthcare workers
Healthcare workers are among the high-risk groups; besides being responsible for infection control and proper patient care, therefore; they must adopt effective prophylaxis-prevention techniques. They should use standard droplet infection control protocols at all times; they should also adopt proper hand and cough hygiene protocols. Waste disposal, utensils, and laundry protocols should be revised and proper policies adopted. Thorough environmental cleaning and handling of patient care equipment should be performed and supervised. Precautions of specimens’ handling and transport should be clear and followed strictly. Observation, strict discipline of family or patient visitors during visiting hours, and limiting their number should be monitored by health care staff. Observing patients’ commitment to proper hygiene and infection control protocols during transport between hospital facilities (X-ray, lab …). Patient support and care are always a primary priority of healthcare workers that should never be compromised in any situation (WHO interim guidance, June 2009).
Concluding remark: Prevention of 2009 H1N1 epidemic- an all society task
Because of the considerable social and economic impact of the pandemic, the WHO report (2009) on prevention of H1N1 2009 pandemic suggested the efforts of governments; businesses, and civil society should work cooperatively to develop prevention plans. Since both impact and duration of the pandemic are unknown, authorities at all levels (local, national, regional, and global) should be prepared to respond to the pandemic effectively. Global and national levels should provide leadership to local and regional authorities, which in turn should be ready to take specifically planned actions. The report highlighted the importance of prevention among workers in vital sectors (Energy, water, healthcare…) as they use materials that might be handled by others, as well as their wide spectrum of mixing with the public. The report also stressed preparing scenarios for different possible situations affected by the vulnerability and capacity to respond. In all cases, plans and protocols should be in line with ethical and legal considerations and fundamental human rights.
References
Boughton, B (2009). Severe H1N1 Infection in Children Linked to Asthma and Other Diseases. 49th Interscience Conference on Antimicrobial Agents and Chemotherapy, San Francisco, CA. 2.
CDC (Disease Control and Prevention Center) (2009). Update: Drug Susceptibility of Swine-Origin Influenza A (H1N1) Viruses, 2009. MMWR, 58, 1-2.
CDC (Disease Control and Prevention Center) (2009). Monovalent Influenza Vaccine Dosage, Administration, and Storage.
Cheng, A. A, Dwyer, D. E, Kotsimbos, A. T. C. et al (2009). ASID/TSANZ guidelines: treatment and prevention of H1N1 influenza 09 (human swine influenza) with antivral agents. MJA, 191, 1-8.
Chowell, G, Bertozzi, S., M, Colchero, M., A. et al (2009). Severe Respiratory Disease Concurrent with the Circulation of H1N1 Influenza. N Engl J Med, 361, 674-679.
Dhamija, P, Bhalla, A, and Medhi, B (2009). Swine Influenza Flu (H1N1 Virus): Therapeutic-Prevention Options and Guidelines. JK Science, 11(4), 181-182.
Holmes, F, Macaulay, R, and Steinbrecher, J (2009). Laboratory Diagnosis of Novel Influenza A (H1N1) and the Limitations of Rapid Influenza diagnostic tests. LabNotes, 17(2), 1-2.
Garcia-Garcia, L, Valdespin-Gomez, J. L, Jimenez-Corona, A. et al (2009). Partial protection of seasonal trivalent inactivated vaccine against novel pandemic influenza A/H1N1 2009: case-conrol study in Mexico City. BMJ, 339, b3928.
Gaydos, J. C, Hodder, R. A, Top, F. H. Jr. et al (1977). Swine influenza A at Fort Dis, New Jersey (January-February 1976). II. Transmission and morbidity in units with cases. J Infect Dis, 136 (Suppl.), 363-368.
Greenberg, M. E, Lai, M. H, Hartel, G. F. et al (2009). Response after One Doe of a Mnovalent Influenza A (H1N1) 2009 Vaccine-Preliminary Report. N Engl J Med, 361 (10.1056/NEJMoa0907413).
Kaore, N. M, Kaore, S. N, Sharma, P. et al (2009). Laboratory Diagnosis of Novel H1N1 Virus. JK Science, 11(4), 172-174.
Kunisaki, K. M, and Janoff, E. N (2009). Influenza in immunosuppressed populations: a review of infection frequency, morbidity, mortality, and vaccine response. Lancet Infect Dis, 9, 493-504.
Kuo, J-S, Lee, Y-H, Hsieh, J-W. et al (2009). Initial Evaluation on Screening of Novel Influenza A (H1N1) at International Ports in Taiwan. Taiwan Epidemiology Bulletin, 25(9), 626-647.
Myer, K. P, Olsen, C. W, and Gray, G. C (2007). Cases of swine influenza in humans: a review of the literature. Clin. Infect Dis, 44, 1084-1089.
Nadkar, M. Y, Subramanian, S, and Ingole, N (2009). H1N1 Influenza: An Update. JAPI, 57, 454-458.
Nelson, M. I, Viboud, C, Simonsen, L. et al (2008). Multiple Reassortment Events in the Evolutionary History of H1N1 Influenza A virus since 1918. PLoS Pathogens, 4(2), 1-12.
Neumann, G, Noda, T, and Kawaoka, Y (2009). Emergence and pandemic potential of swine-origin H1N1 influenza virus. Nature, 459, 931-938.
Novel Swine-Origin Influenza A (H1N1) Virus Investigation Team (2009). Emergence of a Novel Swine-Origin Influenza A (H1N1) Virus in Humans. N Engl J Med, 360, 2605-2615.
Perez-Padilla, R, de la Rosa-Zamboni, D. de Leon S., P. et al (2009). Pneumonial and Respiratory Failure from Swine-Origin Infleunza A (H1N1 in Mexico. N Engl J Med, 361, 680-689.
Poland, G. A, Jacobson, R. M, and Ovsyannikova, I. G (2009). Influenza Virus Resistance to Antiviral Agents: A Plea for Rational Use. CID, 48, 1254-1256.
Sabharwal, S, Mahajan, A, and Gupta, S. K (2009). Swine Influenza A (H1N1 Virus) Flu or Common Cold. JK Science, 11(4), 170-171.
Saleeby, E, Chapman, J, and Morse, J (2009). H1N1 Influenza in Pregnancy. Obstetrics & Gynecology, 114(4), 885-891.
shah, I (2009). Swine Flu (H1N1) and Pediatric Population. JK Science, 11(4), 177-178.
Sharma, R, and Gupta, A (2009). H1N1 Virus in Immunocompromised Patients. JK Science, 11(4), 179.
Smith, T. F, Burget, E. O. Jr, Dowdle, W. R. et al (1976). Isolation of swine influenza virus from autopsy lung tissue of man. N Engl J Med, 294, 708-715.
Sypsa, V, Hatzakis, A (2009). School closure is currently the main strategy stay to mitigate influenza A (H1N1) v: a modeling study. Eurosurveillance, 14(24), 1-7.
Chen, G-W, and Shih, S-R (2009). Genomic signatures of influenza A pandemic (H1N1) 2009 virus. Emerg Infect Dis, Dec; 2009, [Epub ahead of print].
Potter, C. W (2000). Influenza. In Zuckerman, A. J, Banatvala, J. E, and Pattison, J. R (Ed.), Principles and Practice of Clinical Virology (4th edition) (Chapter 5). Chichester, England: John Wiley & Sons, LTD.
Wentworth, D. E, Thompson, B. L, Xu, X. et al (1994). An Influenza A (H1N1) Virus, Closely Related to Swine Influenza Virus, Responsible for a Fatal Case of Human Influenza. Journal of Virology, 68(4), 2051-2058.
WHO (interim Guidance) (2009). Infection prevention and control in health care for confirmed or suspected cases of pandemic (H1N1) 2009 and influenza-like illnesses.
WHO (2009). Whole-of-Society Pandemic Preparedness: WHO guidelines for preparedness and response in the non-health sector. Geneva: Global Influenza Programme.
Yang, Y, Sugimoto, J. D, Halloran, M. E. et al (2009). The transmissibility and control of pandemic influenza A (H1N1) virus. Science, 326(5953), 729-733.
Zimmer, S. M, and Bruke, D. S, (2009). Historical Perspective – Emergence of Influenza A (H1N1) Viruses. N Engl J Med, 361, 279-285.
Appendix