Introduction
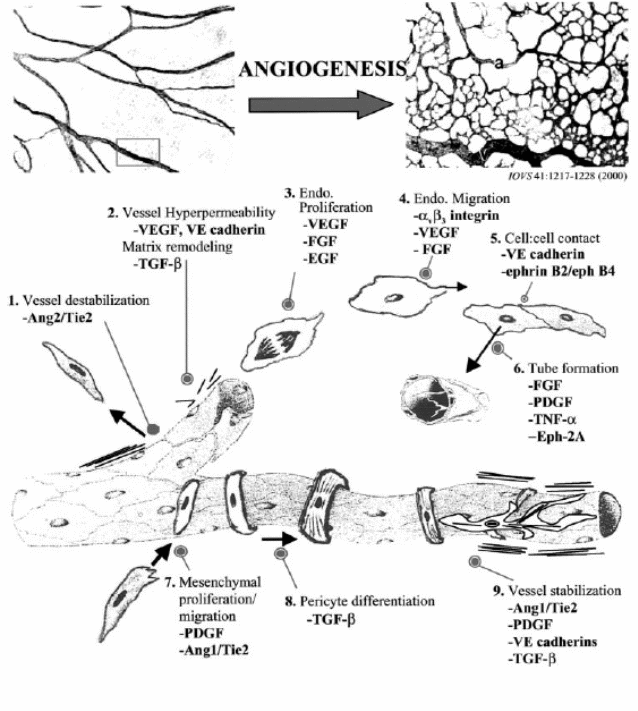
Angiogenesis is the formation of new blood vessels from a microvascular bed. It is a normal multistep process occurring in tissue repair as in wound healing or tissue remodelling as with the development of the placenta. Normally the process is controlled at the endothelial cells’-extracellular matrix interface, a dynamic process of interaction accomplished by targeted cell apoptosis and proliferation. Deposition, stabilisation, and organization of the matrix complete the process. Whereas normal angiogenesis (Figure 1) is a self-limiting process that ends by eliminating hypoxia at the tissue site, in tumour angiogenesis the continuing tumour cell mass hypoxia remains a stimulus. Besides, as tumour vessels fail to mature to normal vasculature, this creates a circle of tumour proliferation, continuous hypoxia, further angiogenesis, leading to further tumour growth. Further, hypoxia regulates the production of pro-angiogenic compounds known as factors of angiogenesis (Papetti and Herman, 2002).
Judah Folkman (after Greenberg and Cheresh, 2008) published the conceptual framework of tumour angiogenesis in 1971. Many experimental laboratories followed this concept in pre- clinical cancer models and more than 30 years later, the FDA approved bevacizumab as the first antiangiogenic drug (Kerbel 2008). In many cancers, there is a disease-free period following treatment (surgical, irradiation or chemotherapy) before metastasis appear during which the patient appears cured. In 1972, Folkman and Gimborne (p. 409) suggested this clinical latency in recurrence might be because of blocked angiogenesis. Later in 2004, Folkman and Kalluri (p. 787) inferred that most tumours arise without angiogenesis remain in situ (dormant non-invasive state) for a variable period. They explained the in situ state is because of host factors preventing tumour transformation to the angiogenic phenotype.
Evidence that tumour growth depends on angiogenesis
Folkman (1990, pp.4-5) suggested that tumor growth depends on angiogenesis that is new vessels appearing around the tumour precede increase in tumour cell population. In this work, Folkman reviewed earlier evidence from experimental work supporting this concept and inferred that growth rate of tumours implanted in experimental animals becomes rapid and logarithmic after vascularisation. Slower growth rates of tumours implanted in avascular region (anterior chamber) than those implanted in vascular areas (iris) suggest that tumour growth depends on angiogenesis. Another evidence was metastatic tumours of retinoblastoma in avascular areas (aqueous or vitrous) are growth restricted and avascular. Radioactive thymidine detection within a solid tumour decreases with increased distance from the nearest open blood vessel. Folkman also suggested that in tumours like malignant melanoma, the appearance of new blood vessels is associated to an increased rate of growth. Folkman (2006, pp. 2-3) stated that a lethal tumour mass can not be produced by tumour cells proliferation without angiogenesis, if this occurs a carcinoma in situ results. Further, Folkman (2006) suggested that oncogene transfer into tumour cells increases angiogenic activity through increasing the expression of VEGF (endothelial growth factor) and decreasing the antiangiogenic proteins expression (like thrombospondin-1). Folkman agreed that oncogene transfer increases tumor mass through increasing tumour cell proliferation over cell death; however, there is reciprocal increased angiogenic activity. Folkman inferred that complete blockade of the angiogenic component of an oncogene will result in a residual microscopic tumour.
Carmeliet and Jain (2000, p. 245) suggested as in any other organ or tissue, malignant cells need oxygen and nutrient and therefore blood vessels to grow. However, those new vessels show structural and functional abnormalities because of persistent angiogenic factors produced by the growing tumor. They inferred that malignant transformation of in situ tumours, or early stages carcinogenesis link to what they called the angiogenic switch. In an extensive review, Jain (2003) reviewed a vast number of references displaying experimental evidence supporting the hypothesis of tumor progression depends on angiogenesis.
Role of angiogenesis in tumor metastasis
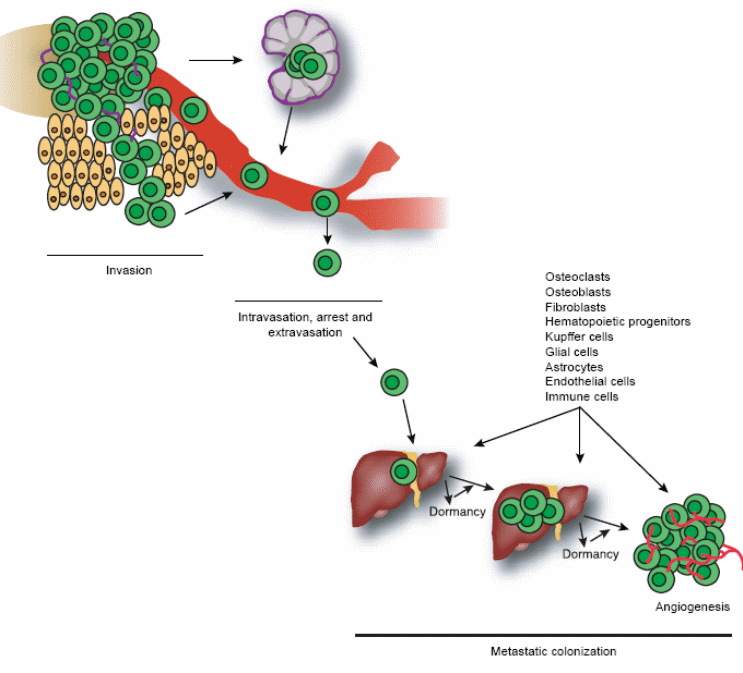
Tumor metastasis consists of a series of biological processes that cause tumor cells to move from the primary neoplasm to a distant location. Tumor cells must invade the tissue surrounding the primary tumor, enter either the lymphatic or blood vessels, survive and ultimately arrest in the circulation, take their way out into a tissue and grow at the new site (seed and soil concept). Thus, the combined influences of tumor cell proliferation, apoptosis, dormancy and angiogenesis in the formation of a progressively growing lesion in a distant site control the process of metastasis (figure 2). The stage of invasion, initial phase of the metastatic process, is characterised by changes in tumour cells adherence to cells of extracellular matrix, which is mediated by integrin (transmembrane protein). Then survival and arrest in the blood stream, tissue colonization follows where angiogenesis is essential for metastasis growth beyond the limits of perfusion. In this case, angiogenesis is stimulated by the angiogenic switch when the ratio of angiogenesis inducers as VEGF, and bFGF favours inhibitors as thrombospondin and endostatin (Steeg, 2006). In the liver, metastatic tumours can be either replacement (non-angiogenic) where tumour cells replace liver cells at the tumour-liver interface keeping tissue architecture or a pushing type influenced by angiogenesis (Takeda et al, 2002).
Role of E-cadherin in tumor metastasis
E-cadherin stands for endothelial cells calcium dependent adhesion molecules, a trans-membrane protein that mediates endothelial cells adherence. It is important for the compilation of vascular structures during angiogenesis and maintaining vascular integrity; therefore, it is a significant target for function-blocking angiogenesis (Dejana, 2004). Studies show that E-cadherin inhibitors inhibit angiogenesis, tumour growth and metastasis in mouse models, besides some E-cadherin inhibitors selectively bind to tumour vasculature to inhibit angiogenesis in pre clinical models, while others inhibit junction formation (May et al, 2006).
Role of MMPS in tumor metastasis
A major function of fibroblasts is to produce extracellular matrix and release matrix metalloproteinases (MMPS) responsible for selective degradation and organization of extracellular matrix. Thus, MMPS are important for the initial phase of metastasis (invasion) (Clark, 1996). The work of Harper and colleagues (2006, p.837) on tumour dormancy animal models enlightened the role of MMPS as participants in the angiogenic switch for both primary and metastatic tumours.
Role of integrin in tumour metastasis
Integrins are heterodimeric (composed of two similar but not identical molecules or monomers) transmembrane glycoproteins whose function is to mediate cell-cell or cell-extracellular matrix attachments. This takes place r through either attachment to basement membrane or to cell ligands (binding to a central atom of a molecule forming a complex). Integrin binding triggers many aspects of cell behaviour including survival, proliferation, and control of transcription, motility and cytoskeletal organization. Integrins participate in promoting endothelial cells migration and survival as focal adhesions linking Integrins to actin microfilaments act like scaffolds that promote cell invasion and migration. Thus, they contribute to tumour progression and to metastasis (Garmy- Susini and Varner, 2008).
Main angiogenesis regulators (Factors)
Angiogenesis occurs in four sequential steps; first proteases degrade the basement membrane, then migration and sprouting of endothelial cells into the interstitial space followed by proliferation of endothelial cells at the tip of the sprouting mass. Finally is lumen and anastmosis formation to allow blood flow (Plank and Sleeman, 2003). Angiogenic phenotype serves the development of malignant tumours at different stages, and a change balance between positive (angiogenesis enhancing) and negative (angiogenesis inhibiting) regulators of vessels growth in the tumour microenvironment causes switch to the angiogenic phenotype. An understanding of the different regulators whether positive or negative can be enlightened based on the various factors’ involvement in tumour angiogenesis (Gupta and Qin, 2003).
Angiogenesis enhancing factors (positive regulators)
Vascular endothelial growth factor (VEGF)
VEGF is also known as vascular permeability factor (VPF), it is a heparin binding angiogenic factor expressed in many tumours. There are three possible mechanisms of its action; first, it may increase endothelial cells permeability by enhancing the activity of vesicular organelles present in endothelial cells lining small vessels; thus, helps the transfer of metabolites across plasma membranes. Second, it may act by enhancing permeability through mitogen-activated protein kinase (MAP kinase) resulting in loosening adhering forces between endothelial cells (rearrangement of cadherin complexes). Alternatively, it may enhance permeability through activating nitric oxide synthetase (Gupta and Qin, 2003). In addition, activation of the VEGF-VEGF receptor pathway stimulates a complex of signalling processes supporting endothelial cells growth, migration and survival from pre existing vasculature (Hicklin and Ellis, 2005).
Fibroblast growth factor (FGF)
There are nine-fibroblast growth factors acting on a family of four-tyrosine kinase transmembrane receptors. Although evidence shows they are well- expressed in many types of tumours’ cells and are essential angiogenesis and endothelial cells survival factors, yet the exact mechanism is open for research (Folkman, 2003). Tonini and colleagues (2003, pp.6549) suggested as many FGFs contain signal peptides secreted in the extracellular matrix where they bind with heparin- like glycosaminoglycans (HLGAGs). From this point, may act either directly on target cells or indirectly through a carrier protein. Alternatively, they suggested binding of FGF with their receptors results in activation of many signal transfer flow to induce its role in tumour growth and angiogenesis, progression of steroid hormone dependent cancers to autonomous state of non-dependence.
Other mechanisms suggested are that bFGF induces expression of anti apoptosis proteins, or synergistically enhances VEGF mediated hepatocellular carcinoma and angiogenesis. FGFs may be responsible for producing extracellular matrix and release of MMPS (Folkman, 2003).
Matrix metalloproteinase (MMPs)
The principal concept about MMPs is they enhance endothelial cells migration through the dense extracellular matrix (ECM). They may do this through degradation of basement membrane and ECM, which links to tumour growth, metastasis and angiogenesis. Alternatively, on degrading ECM, they cause the release of mitogens stored, besides, the release of soluble FGF receptor 1 among other growth factors (Nagase et al, 2006). Recent evidence, however, suggests that proteolysis of ECM proteins releases anti angiogenic molecules affecting the bioavailability of angiogenic factors. Thus, MMPs can be considered pro angiogenic (positive factors) and have anti angiogenic activity (negative factors). There are four recently evolved concepts about the role played by MPPs; first is angiogenesis needs a suitable proteolytic balance to take place otherwise defective angiogenesis takes place. Second is the proteolytic activity taking place close to endothelial cells ECM interface is the one that controls angiogenesis. Third, is proteolytic degradation of ECM proteins releases both angiogenesis inhibiting factors, and at the same time it releases and solubilises ECM bound growth factors. Finally, MMPs may target other proteins in addition to ECM proteins (Page-McCaw et al, 2007).
Integrins
Coordinated interaction of endothelial integrins and the extracellular matrix is an essential step in angiogenesis driven by growth factors. Evidence supports that growth factor receptors, integrins interact physically, and functionally to produce signals needed for angiogenesis (Smyth and Patterson, 2002). Hynes and others (1999, p. 501), explained this mechanism suggesting that integrins mediate adhesion of cells to ECM proteins and perhaps to other cells, and that growth factors like VEGF control cell surface expression of integrins. They suggested that integrins mediate cell adhesion and migration on many ECM molecules needed for vascular development like fibronectin, vitronectin, thrombospondin, and entactin. They suggested another mechanism relevant to angiogenesis that integrins also mediate intracellular signalling controlling features of cytoskeletal organisation and cell motility. Thus, since integrins regulate both cell-cell and cell to matrix contacts, they participate in neoangeogenesis (Garmy-Susini and Varner, 2008).
Angiogenesis inhibitors
Angiogenesis inhibitors are substances that can inhibit the process of angiogenesis needed for growth and metastasis of malignant tumours. These substances may act by one or more of the following mechanisms; first, they may inhibit tumour cells from producing angiogenic-enhancing proteins. Second, they can block receptor-angiogenic protein interaction; third, they may neutralize the angiogenic protein, or finally, they may act directly on the tumour endothelial cells inhibiting their proliferation and migration. These substances can be endogenous and significantly increased by medications, or exogenous in the form of medications or food that contains antiangiogenic elements (Folkman, 2004).
Endogenous inhibitors
Endogenous angiogenesis inhibitors include many antiangiogenic peptide, hormone metabolites and apoptosis modulators, and are classified into matrix-derived and non-matrix derived. Vascular basement membrane components like type IV collagen provide mesh-like structural support in conjunction with other macromolecules as heparan sulphate, laminin, and entactin. In addition, such components can alter endothelial cells behaviour. Non-matrix derived inhibitors can block vascular invasion and tumour growth like chondromodulin-I derived from cartilage. Alternatively, they may inhibit endothelial cells proliferation and migration like angiostatineen resulting from action of MMPs on plasmin (Nyberg et al, 2005).
Levchenko and others (2008, p. 880) suggested that some endogenous angiogenesis inhibitors act directly on endothelial cells making them unresponsive to mitogenic and migratory signals. Currently there are more than 27 proteins known as endogenous angiogenesis inhibitors most of them are proteolysis products of larger precursor proteins. Although stimulating them to counteract the proangiogenic factors might represent a promising line of therapy; yet, how precisely they coordinate the formation of this counteracting barrier is not fully understood (Nyberg et al, 2005).
Angiostatin is an example of non-matrix derived endogenous angiogenesis inhibitors. It is an enigmatic derivative of plasminogen, which does not have similar inhibitory characteristics. It suppresses endothelial cells migration and proliferation, and enhances endothelial apoptosis through acting on TNF-α mediated endothelial cells apoptosis. It also inhibits tube formation associated with proliferating endothelial cells. It inhibits in vivo growth of Lewis lung metastasis (Carmeliet, 2000).
Endostatin is an example of matrix-derived endogenous angiogenesis inhibitors. Like angiostatin, it is a proteolytic derivative of collagen XVIII available at the basement membrane and vessel walls. In vitro studies show it is endothelial cells specific, as it does not influence other tumour cells. Its endothelial cell death effects link to reduced expression of anti apoptotic proteins (Bcl-2 and Bcl-XL), which are protective against programmed cell death. Another mechanism of action is the activation of Capspase-3, an intracellular protease that starts cell death by degrading certain DNA repair proteins (Lee Sim et al, 2000). Boehm and others (1997, p. 404) showed that endostatin affects fibrosarcoma and melanoma murine tumour models dependent on angiogenesis for growth.
Thrombospondin is a multifunctional extracellular matrix glycoprotein that controls cell adhesion and proliferation, angiogenesis, and some growth factors (TGF-h) and proteases activation. Some studies suggest that thrombospondin may possess both positive and negative angiogenic properties depending on the protease generating the thrombospondin fragments (Nyberg et al, 2005). Hoekstra and colleagues (2005, p.5188) stated that thrombospondin inhibits VEGF-induced corneal neovasculariztion in experimental animals. They showed successful phase I trials of ABT-510, a derivative of thrombospondin-1 in controlling malignant progression of melanoma, breast and lung carcinomas.
Angiopoietins function as ligands for Tie2/Tek tyrosine kinase receptors expressed on vascular endothelium. Significant Tie2 expression has been shown in non-small cell lung cancer, hepatocellular, prostatic carcinomas and Kaposi sarcoma (Kobayashi and Lin, 2005). There are two members of angiopoietins family, angiopoietins-1, which maintains mature vessels by enhancing the interaction between endothelial and supporting cells. Angiopoietin-2 is expressed at sites of vascular remodelling and it is role is believed to counteract the stabilizing effect of angiopoietin-1 (Moon et al, 2006).
Exogenous inhibitors
Exogenous angiogenesis inhibitors are compound designed to target a specific site or mechanism of angiogenesis. They may inhibit endothelial cells proliferation and migration; inhibit the production or release of angiogenesis mediators like VEGF and FGF-2. They may also act through interfering with tyrosine kinase receptor signalling, or down-regulate the expression of certain proangiogenic factors. Another possible mechanism is targeting enzymes with angiogenic characteristics like PD-ECGF/TP and COX. PD-ECGF (Liekens et al, 2001).
Bevacizumab (avastin) is a humanised monoclonal antibody specific against vascular endothelial growth factor (VEGF) neutralizing it before combined with its receptors on endothelial cells. Thus, it is a type I angiogenic inhibitor (blocking a single angiogenic protein). The FDA approved the drug for treatment of patients with metastatic colon carcinoma in 2004 (Folkman, 2004). In addition, clinical trial shows it is effective either alone or in combination with chemotherapy for a number of other malignancies like non-small cell lung cancer, metastatic breast cancer, pancreatic and renal cancer (Ferrara et al, 2005).
SU5416 is another VEGF inhibitor specific to tyrosine kinase receptors (VEGF TK inhibitor) that showed efficacy in to manage solid tumours. Clinical trials showed SU5416 is effective against solid tumours like renal cell carcinoma, melanoma and soft tissue sarcoma. Clinical trials when combined with chemotherapy did not show successful results, also its toxicity profile is undesirable (Kantarjian and Karp, 2003).
Vitaxin is a humanised monoclonal antibody blocking av b3 integrin receptors. It is a direct angiogenesis inhibitor agent preventing endothelial cells from responding to proangiogenic factors. Current clinical trial include using vitaxin in combination with other chemotherapeutic agents in late cases of malignant melanoma and cases of cancer prostate with bone metastasis (Posey et al, 2001).
Marimastat is an exogenous synthetic metalloproteinase inhibitor (MMPs inhibitor). Clinical trials show it has the advantage of being administered orally and is on trial for advanced case of non-small cell lung cancer and prostate cancer (Goffin et al, 2005).
TNP-470 is a synthetic derivative of antibiotic fumagillin, which blocks methionine aminoterminal processing needed for endothelial cells growth. It affects in vitro endothelial cells growth and migration and shows animal models and clinical trials effectiveness with solid tumours as well as acute leukaemia and lymphomas (Liekens et al, 2001).
CM101 is an exotoxin (polysaccharide) produced by β streptococcus targeting pathological neovascularisation by activating complement C3. Thus, it induces inflammatory infiltration of pathological neovascularisation resulting in inhibition of tumour growth and tumour cells apoptosis. Murine tumours models and phase I clinical trials show that CM-101 does not bind to leukocytes, normal cells, or directly to tumour cells with no apparent biological effect on these cells (Yakes et al, 2000).
A word on positive and negative angiogenetic factors
The onset of tumour angiogenesis depends on a shift of balance of the positive and negative angiogenesis regulators, which may take place in the tumour cells, or between tumour cells’ angiogenic proteins and the host antiangiogenic proteins (Folkman, 2003). Table 1 shows the negative and positive regulators of angiogenesis based of data derived from Folkman (2003), and Gupta and Qin (2003)Table 1: Negative and positive regulators of angiogenesis:
Foods that contain antiangiogenic elements
Flavonoids are available in fruits, vegetables and beverages like green tea and red wine, their health value has been recognised since the knowledge of the French Paradox. Their anti tumour influence is still open to discussion. Quercetin and apigenin, which account for over 95% of falvonoids intake inhibit melanoma growth and affect metastatic potential in murine animal models. Some research suggests these compounds may influence tumour angiogenesis, the weak evidence points to targeting protein kinases (Nijveldt et al, 2001).
Conclusion
Angiogenesis has been shown essential to solid tumours growth, switching the status from in situ to growing and metastasising. There are three basic activities of endothelial cells in angiogenesis, secretion of proteolytic enzymes, proliferation and migration. Proangiogenic and anti angiogenic regulators control these activities. The onset of angiogenesis depends on disruption of this balance. Targeting malignant growth and metastasis can be through stimulation of endogenous inhibitory regulators or the administration of exogenous inhibitory regulators.
References
- Akihiko, T., Stoeltzing, O., Ahmad, S. A., Reinmuth, N. et al, 2002. Role of Angiogenesis and Growth of Liver metastasis. Annals of Surgical Oncology, 9(7), 610-616.
- Boehm, T., Folkman, J., Broweder, T., and O’Reilly, M. S., 1997. Antiangiogenic therapy of experimental cancer does not induce acquired drug resistance. Nature, 390, 404-407.
- Carmeliet, P., 2000. Mechanisms of angiogenesis and arteriogenesis. Nature Medicine, 6(3), 389-395.
- Carmeliet, P., and Jain, R. K., 2000. Angiogenesis in cancer and other diseases. Nature, 407, 249-257.
- Clark, R. A. F., 1996. Wound repair: overview and general considerations. In Clark, R. A. F., ed. Molecular and Cellular Biology of Wound Repair. 2nd edition. New York: Plenum. 1-50.
- Dejana, E., 2004. Endothelial cell-cell junctions: happy together. Nat Rev Mol Cell Biol, (5), 261-270.
- Ferrara, N., Hillan, K. J., and Novotny, W., 2005.., Bevacizumab (Avastin), a humanized anti-VEGF monoclonal antibody for cancer therapy. Biochem Biophys Res Commun, 333(2), 328-335.
- Folkman, J., 1972. Anti-angiogenesis: new concept therapy of solid tumors. Ann Surg, 175(3), 409-416.
- Folkman, J., 1990. What Is the Evidence That Tumours Are Angiogenesis Dependent? Journal of the National Cancer Institute, 82(1), 4-6.
- Folkman, J., 2003. Fundamental Concepts of the Angiogenic Process. Current Molecular Medicine, (3), 643-651.
- Folkman, J., 2004. Endogenous angiogenesis inhibitors. APMIS, (112), 496–507
- Folkman, J., 2006. Angiogenesis. Annu. Rev. Med., (57), 1-18.
- Folkman, J., and Kalluri, R., 2004. Cancer without disease. Nature, (42), 787.
- Garmy-Susini, B., and Varner, J. A., 2008. Roles of Integrins in Tumor Angiogenesis and Lymphangiogenesis. Lymphatic Research and Biology, 6 (3-4), 155-163.
- Greenberg, J. I., and Cheresh, D. A., 2008. Antiangiogenic Cancer Therapy. NEJM, 359(5), 545-546.
- Gofin, J. R., Anderson, I. C., Supko, J. G., et al, 2005. Phase I trial of the matrix metalloproteinase inhibitor marimastat combined with carboplatin and paclitaxel in patients with advanced non-small cell lung cancer. Clin Cancer Res, 11(9), 3417-3424.
- Gupta, M. K. and Qin, Ren-Yi, 2003. Mechanism and its regulation of tumor-induced angiogenesis. World J Gastroenterol, 9(6), 1144-1155.
- Harper, J., Naumov, G. N., Exarhopoulos, A., Bender, E. et al, 2006. Predicting the switch to the angiogenic phenotype in a human tumor model. Proc Am Assoc Cancer Res, (47), 837a.
- Hicklin, D. J. and Ellis, L. M., 2005. Role of the Vascular Endothelial Growth Factor Pathway in Tumor Growth and Angiogenesis. J Clin Oncol, (23), 1011-1027.
- Hoekstra, R., de Vos, F. Y. F. L., Eskens, F. A. L. M., et al, 2005. A phase I safety pharmacokinetic and pharmacodynamic study of the thrombospondin-1 mimetic angiogenesis inhibitor ABT-510 in patients with advanced cancer. J Clin Oncol, (23), 5188-5197.
- Hynes, R. O., Bader, B. L., and Hodivala-Dilke, K., 1999. Integrins in vascular development. Brazilian Journal of Medical and Biological Research, (32), 501-510.
- Jain, R. K., 2003. Molecular regulation of vessel maturation. Nat Med, (9), 685-693.
- Kantarjian, H. M., and Karp, J. E., 2003. SU5416, a small molecule tyrosine kinase receptor inhibitor, has biologic activity in patients with refractory acute myeloid leukemia or myelodysplastic syndromes. Blood, 102, 795-801.
- Kerbel, R. S., 2008. Tumor Angiogenesis. NEJM, 358(19), 2039-2049.
- Kobayashi, H., and Lin, P. C., 2005. Angiopoietin/Tie2 signaling, tumor angiogenesis and inflammatory disease. Frontiers in Bioscience, 10, 666-674.
- Lee Sim, B. K., MacDonald, N., J., and Gubish, E. R., 2000. Angiostatin and Endostatin: Endogenous inhibitors of tumor growth. Cancer and Metastasis Reviews, 19, 181-190.
- Levchenko, T., Veitonma, K., Lundkvist, A., Gerhardt, H. et al, 2008. Therapeutic antibodies targeting angiomotin inhibit angiogenesis in vivo. FASEB J, (22), 880-889.
- Liekens, S., De Clercq, E., and Neyts, J., 2001. Angiogenesis: regulators and clinical applications. Biochemical Pharmacology, (61), 253-270
- May, C., Doody. J. F., Abdullah, R., Balderes, P. et al, 2005. Identification of a transiently exposed VE-cadherin epitope that allows for specific targeting of an antibody to the tumor neovasculature. Blood, 105(11), 4337-4344.
- Moon, W. S., Park, H. S., Yu, H. K. et al, 2006. Expression of Angiopoietin 1, 2 and Their Common Receptor Tie2 in Human Gastric Carcinoma: Implication for Angiogenesis. J Korean Med Sci, 21, 272-278.
- Nagase, H., Visse, R., and Murphy, G., 2006. Structure and function of matrix metalloproteinases and TIMPS. Cardiovascular Research, (69), 562-573.
- Nijveldt, R. J., van Nood, E., van Hoorn, D. EC., et al, 2001. Flavonoids: a review of probable mechanisms of action and potential applications. Am J Clin Nutr, 74, 418-425.
- Nyberg, P., Xie, L., and Kalluri, R., 2005. Endogenous Inhibitors of Angiogenesis. Cancer Res, 65(10), 3967-3979.
- Page-McCaw, A., Ewald, A, J., and Werb, Z., 2007. Matrix metalloproteinases and the regulation of tissue remodelling. Nature Review-Molecular Cell Biology, (8), 221-233.
- Papetti, M., and Herman, I. M., 2002. Mechanisms of tumor-derived angiogenesis. Am J Physiol Cell Physiol, (282), C947-C970.
- Plank, M. J. and Sleeman, B. D., 2003. Tumour-induced Angiogenesis: A Review. Journal of Theoretical Medicine, 5(3-4), 137-153.
- Posey, A. J., Khazaeli, M. B., Del Grosso, A., et al, 2001. A pilot trial of Vitaxin, a humanized anti-vitronectin receptor (anti-aVb3) antibody in patients with metastatic cancer. Cancer Biother Radiopharm, 16, 125-132.
- Smyth, S. S., and Patterson, C., 2002. Tiny dancers: the integrin-growth factor nexus in angiogenic signaling. The Journal of Cell Biology, (158), 17-21.
- Steeg, P. S., 2006. Tumor metastasis: mechanistic insights and clinical challenges. Mature Medicine, 12(8), 895-904.
- Tonini, T., Rossi, F., and Claudio, P. P., 2003. Molecular basis of angiogenesis and cancer. Oncogene, (22), 6549-6556.
- Yakes, F. M., Wamil, B. D., Sun, F. et al. 2000. CM101 Treatment Overrides Tumor-induced Immunoprivilege Leading to Apoptosis. Cancer Research, 60, 5740-5746.