Introduction
The brochure outlines the use of Artesunate drug which is used in the treatment of severe malaria among adults and pediatric patients. Severe malaria is caused by plasmodium falciparum according to Khanal (2021) and is characterized by extreme fevers, severe headaches, nausea and vomiting, diarrhea, muscle and joint pains, and chills. The targeted audience for the brochure is the patients suffering from malaria, their social support system, and family and also clinical researchers. The brochure aims at enlightening patients and researchers about artesunate drugs, their chemical composition, administration, side effects, and action mechanism.
Drug information
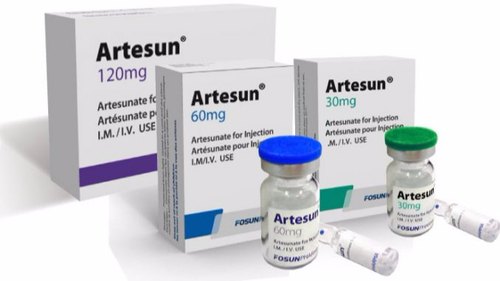
Artesunate is a drug found in the artemisinin class of medications. According to Khanal (2021), artesunate’s mode of action spells that the drug is metabolized in the DHA whereupon the DHA’s endoperoxide bridge reacts with heme resulting in free radicals which inhibit proteins and nucleic acid synthesis. Artesunate is a white powder administered through an injection as shown in Figure 1 below intravenously or intramuscularly in 2.4 mg at 0 hours, 12 hours, and 24 hours for patients above 20 kilograms (Kitabi et al., 2021). For children below 20 kilograms, the dosage should be 3mg at 0, 12, and 24 hours. Artesunate drug is likely to lead to yellowing of skin and eyes, hemoglobinuria, and renal failure. Artesunate interactions mention the precautions on taking artesunate and some of the anticipated side effects.
Patient Education Considerations
The healthcare practitioners first assess the needs or possible treatment of severe malaria by artesunate drug. The offering of patient education by the health care practitioners educates patients, their caregivers, and family on the medication’s instructions to be taken as directed by the physician. Patient education on usage or artesunate encompasses the adverse effects of the drugs and how to counteract them. In cases of developed allergic reactions, the medication’s usage is discontinued.
References
Khanal, P. (2021). Antimalarial and anticancer properties of artesunate and other artemisinins: current development. Monatshefte für Chemie-Chemical Monthly, 152(4), 387-400.
Kitabi, E., Bensman, T. J., Earp, J. C., Chilukuri, D. M., Smith, H., Ball, L.,… & Reynolds, K. S. (2021). Effect of Body Weight and Age on the Pharmacokinetics of Dihydroartemisinin: Food and Drug Administration Basis for Dose Determination of Artesunate for Injection in Pediatric Patients With Severe Malaria. Clinical Infectious Diseases, 73(5), 903-906.