Objective
The aim of this experiment was twofold: to demonstrate that highly complex alcohol reactions could be described using acquired knowledge and to show how reposition of a vicinal diol using the movement of a methyl compound could produce a new substance, a ketone.
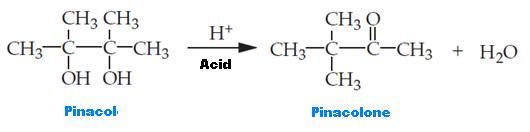
Synthesis equation for the reaction
Physical properties
Pinacol
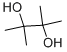
Molar mass: 118.17 g/mol
Melting point: 40-43 °C
Boiling point: 171-172 °C
Density: 0.967 g/mL
Hazards: Irritant, flammable. (1)
Water
Molar mass: 18.01 g/mol
Melting point: 0 °C
Boiling point: 100 °C
Density: 0.997 g/mL (2)
Sulfuric acid
Molar mass: 98.08g/mol
Melting point: 10 °C
Boiling point: 290 °C
Density: 1.840g/mL (3)
Hazards: Toxic, flammable, corrosive, and irritant.
Sodium chloride
Molar mass: 58.44g/mol
Melting point: 801 °C
Boiling point: 1413 °C (4)
Density: 2.17g/mL
Hazards: Irritant.
Sodium sulfate
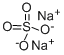
Molar mass: 142.04g/mol
Melting point: 884 °C
Boiling point: 1700 °C
Density: 2.68g/mL (5)
Hazards: Irritant.
Pinacolone
Molar mass: 100.16g/mol
Melting point: -52.5 °C
Boiling point: 106°C
Density: 0.801g/mL
Hazards: Harmful, flammable, and irritant. (6)
For the 2,4-DNP test:
2,4-dinitrophenylhydrazine
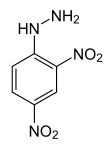
(2,4-DNP):
Molar mass: 198.14g/mol
Melting point: 197-200 °C
Density: 0.843g/ml
Hazards: Flammable, harmful
Procedure
The experiment was initiated with a precise 3.0 g of Pinnacol and another 30 ml of deionized water were placed into a 50 ml round-bottom flask and gently heated using a heating mantle until the mixture disintegrated. The content of the round-bottom flask was then cooled to room temperature using a tap water bath, and 2 boiling chips were added. Further, the content was gently and gradually swirled, and then 0.5 ml of concentrated sulfuric acid was added. The hot flask was cooled in a tap water bath as the temperature rose, and the mixture was further swirled for 10 minutes.
The subsequent process involved distillation in which a simple distillation instrument was connected to the round-bottom flask followed by distillation into a 25 ml round-bottom flask. Once the temperature of the vapor rose to 100 °C, the distillation process was haltered. The distillate was then mixed with 5 ml of saturated sodium chloride. A transfer pipet was then used to separate the aqueous layer of the content while anhydrous sodium sulfate was used to dry the organic layer. A dry, clean, and pre-weighed sample flask with a tight cap was used to store the dried component following the decantation. A percent yield was determined after weighing the stored liquid and further analysis of IR and the 2,4-DNP were conducted on the substance. The capped substance was then stored in the laboratory for the next lab process that involved the assessment of the NRM.
Calculation of theoretical and percent yield
Theoretical yield:
- Mass of Pinnacol= 3.0 g
- Moles of Pinnacol= 3.0g/ 118.17 g/mol= 0.02538 mol
- Theoretical yield=(0.02538mol*100.16g/mol)/ 1 mol of pinacolone= 2.542g
Percent yield:
- Empty sample vial= 36.3g
- Sample vial + content= 38.02g
- Mass of the product= 1.72 g
- Percent yield= (Actual/theoretical) *100= (1.72g/2.542g)*100= 67.66%
Synopsis of results
Following the deposition of the pinacol weighted 1.72 g, the substance that was acquired was pinacolone. The determine theoretical yield was 2.542 g. Additionally, the percent yield of pinacolone substance was 67.66%. Multiple explanations can be used to account for the noted modest result of penacolone. The first relevant possibility is that not all constituents of the pinacol were repositioned to form pinacolone and some constituents could have remained in their original form as reactants. On the contrary, the IR spectrum was different. That is, the spectrum did not indicate any peak at the range of 3500-3600 cm-1 ((-OH) stretch), implying that no residual pinacol was available after the experiment. The less percent yield could also be attributed to possible evaporation of the pinacol constituents because of high temperatures that reduced the number of pinacol constituents for repositioning into pinacolone. Finally, the modest yield could also be attributed to other factors that were difficult to manage.
Discussion
The reaction for pinacol reposition is classified as a dehydration reaction. The dehydration reaction usually involves various 1,2-diols (glycols). This reaction derives its name from the reactant pinacol that is changed to pinacolone. Through an SN1 reaction mechanism, the reposition takes place by ensuring that a carbonyl element is transformed from a diol. The reaction was catalyzed by acid because alcohols are considered as poor separation groups. The catalyst concentrated sulfuric acid (H2SO4) was necessary to protonate a given alcohol element to create a better separating group. The reaction was exothermic, and the tap water bath rather an ice bath was used to cool down the round-bottom flask to avoid recrystallization. During protonation of oxygen found in the hydroxyl compound, the donation of a couple of electrons to H+ occurs and new oxygen is added to the hydrogen bond. This process transforms the alcohol into an oxonium species (-OH2+) that is considered a better separating group.
Stable tertiary carbonation results from the water loss and the relatively stable carbonation might be changed into further stable carbonation through the movement of a methyl element (methyl shift). The added stability results from the placed positive charge on the carbon in which the second (-OH) element is found. Resonance ensures that other nonbonding electrons of oxygen found on the hydroxyl element are used to stabilize the positive charge. Therefore, following the reaction and deprotonation of the resonance-stabilized cation, pinacolone is produced as the product.
Pinacolone consists of a carbonyl element, which differentiates it from the compound used to start the experiment, the pinacol. Another product in this chemical reaction is water. A simple distillation instrument is installed to co-distill water and pinacolone to isolate the products of this reaction. Notably, pinacolone and water do not mix. Immiscible liquids are normally co-distilled lower than the boiling point of each of the constituent based on Raoult’s principle. Water has a boiling point of 100 °C and, thus, no more distillation is expected to occur after attaining 100 °C because at this point pinacolone is effectively co-distilled with water. Moreover, continued heating of the substance results in the distillation of unreacted pinacol, which is an unwanted outcome. Inorganic impurities and excess water are separated using sodium chloride, leading to the separation of the two layers. Two layers are realized following distillation because the two substances have various densities. The organic pinacolone makes up the upper layer due to its low density of 0.801 g/mL relative to the aqueous water layer with a density of 0.997 g/mL. To ensure the integrity of the IR and the NMR spectra analysis, water must be eliminated from the final product. Hence, any available water molecule was removed from the organic layer using anhydrous sodium sulfate.
Some tests were conducted to ensure that the final product was pinacolone. First, the IR spectrum is used to illustrate the reaction and demonstrate how pinacol (with (-OH) elasticity of about 3500-3600 cm-1) changes to pinacolone. A clear peak is demonstrated at the pinacol’s IR, which shows the presence of the hydroxyl elements. On the contrary, no (-OH) elasticity is observed in the pinacolone spectrum. Nevertheless, it bears a carbon (C=O) about 1700 cm-1, which showed that a ketone was produced during the reposition of pinacol.
Second, two peaks were associated with the NMR spectrum of the pinacolone, which demonstrated that two different protons were present in the substance. They included protons on the tert-butyl and other protons on the methyl elements. The tert-butyl constituent has a relatively higher peak than the peak of methyl elements. This difference is attributed to 9 protons found on the tert-butyl compared to 3 hydrogen protons on the methyl group. It is also noted that the peaks are singlet due to proximity of the methyl protons to the carbonyl constituent, which lacks proton. Further, the carbon that does not have any protons is attached to protons found on the tert-butyl component. A similar observation is noted for the pinacol where split may occur and cause the variation in the size of the peaks. The peak observed at 2.1 ppm pinacol spectrum is a notable variation, and it is moved to the left. One can, therefore, infer that protons, which are openly joined to oxygen in the pinacol, are down-shielded. The lack of movement in the spectrum of the resultant product showed that pinacolone was synthesized.
Finally, a test involving 2,4-DNP was also used to demonstrate the presence of pinacolone. Pinacolone consists of the carbonyl group, and it is the resultant product following the rearrangement reaction of pinacol. An orange solid substance is produced after adding a few drops of the product to 2,4-DNP, which was associated with the existence of a carbon-oxygen double bond found in the ketone (pinacolone). The reaction occurring through condensation involved the reagent 2,4-DNP, which quickly reacted with ketones or aldehydes. The presence of a solitary pair of electrons on the end of the amino group found in the 2,4-DNP reagent ensured that a suitable nucleophile was available. The orange precipitate, 2,4- dinitrophenylhydrazone was obtained following the effect of the nucleophilic 2,4-DNP on the electrophilic carbonyl group of the pinacolone.
References
www.chemicalbook.com
Wade, L.G. Organic Chemistry. Eighth ed. N.p. Pearson, n.