Introduction
During my academic life, I have always observed that companies that use water as a heating medium prefer using steam to boiling water. This tendency has always amazed me since the boiling water and steam have the same temperature which is 100 degree Celsius. I have been arguing that if water and steam have the same temperature, none of them should be preferred to the other on basis of the heating capability.
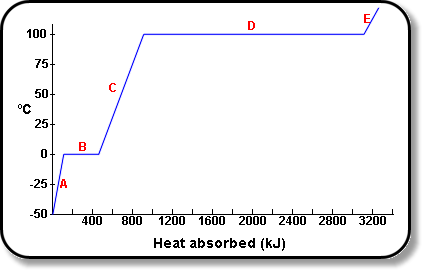
Additionally, I noted that after sweating, I feel cold for a moment although the ambient temperatures are very high. Based on these mysteries of nature I decided to investigate what happens during vaporization of water in order to explain the phenomenon. The figure below is a graph representing the heating curve of pure water where we observe that water maintains 100 degrees Celsius for a moment before turning to steam.
Conceptual Framework
There are concepts and theories that explain why steam is a better heating medium than boiling water although they might have the same temperature. Scientists argue that before substances change from liquids to gas, they absorb heat without showing increase in the temperature. This heat is hidden in a manner that it cannot be identified by increase in temperature implying that they are not even sensed by a thermometer. The hidden heat is referred to as the latent heat of vaporization since it is invisible to apparatus that detect changes in temperature.
When the heat is measured per unit mass, which is mostly considered as one kilogram, the measure is referred to as the specific latent heat of vaporization. Various substances have different amounts of latent heat of vaporization since they have differing complexions. The table 1 shows the various substances along with their corresponding specific latent heat of vaporization.
Table 1: It shows the different substances along with their specific latent of vaporization.
From the table above, it is evident that water has a specific latent heat of vaporization of 2260kJ/Kg implying that the any mass of water absorbs heat that is proportional to this ratio (Lefrois, 1979). When calculating the amount that a mass of substance need to vaporize, we multiply the specific latent heat of vaporization with the mass.
Heat absorbed= Specific latent heat of vaporization x Mass of the vaporizing substance
Q= m x Hv
In this case, Q is the amount of heat absorbed, m represents the mass of substance which will evaporate, and Hv is the specific latent heat of vaporization of the substance. For example, when 0.3 kilograms of alcohol is evaporating it absorbs 256.5 kJ of heat. This is obtained by multiplying 855 kJ/Kg by 0.3 Kilograms.
Q= 855 kJ/Kg x 0.3 Kgs = 256.5 Kilojoules
Risks and Risk Control
There are various risks that are conjoined with this experiment due to the technicality of the experiments. This list below shows the risks and how they will be controlled.
- Since the experiment involves heating water the steam might burn inflicting injuries on the body. Additionally, the hot apparatus might cause burns on the skin leading to pain and wound. In order to control this risk, holders will be used ensuring that the apparatus are not held with bare hands.
- Secondly, the apparatus might break due to when heating especially if they experience temperature imbalances. In this case, hot apparatus that are made of glass will not be washed using cold water before they cold down. Thermometer will be kept in their bags when they are not in use.
- Lastly, spillage of water on the floor might make the floor become wet and lead to slipping that can cause adverse bodily injuries. Lags will be used to wipe the floor and benches ensuring there is not water on the floors.
Experiment Procedures and Requirements
This experiment needs an electrical heater, a boiling trough, one stop watch, and a balance for measuring mass.
Procedure
- Set up the tools that are described above as shown in figure 2 in order to set for the experiment.
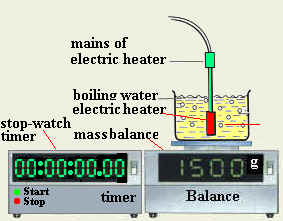
- Measure the original mass of the boiling trough, water and the heater. Record this mass as M1.
- Identify the heater’s watt and record it as W.
- Immerse the heater in the water and put on the heater
- Start the watch when the water starts boiling and not before it boils.
- Record the mass of the trough and water after every three minutes. This should be recorded on the M2 column.
- The time should be recorded besides the time column
- Record the data in table 2 which is shown below.
- M1=
- W=
- Calculate the mass of evaporated liquid by subtracting M2 from M1 and record this on the corresponding column.
During calculation, it should be considered that the heat released during by the heater is equal to the heat that is absorbed by the water and trough.
(Wheater) x Time = m x Hv.
Therefore, the specific latent heat of vaporization can be obtained by making the subject of the above formula and compute the result.
Hv = ((Wheater) x Time)/m
Results Obtained and Analysis
M1=1030 g
W= 126 J/s
Taking the first data set of the results that were obtained we can calculate the latent heat of vaporization.
P= W x t
P= 126 x 180s = 22680
Hv = 22680/ 10= 2268 j/Kg
We can find the average by adding all the value of latent heat and dividing by three. (2268+2061+1890)/3= 2073 J/Kg
This value could be compared with the theoretical value which is 2260 J/Kg. However, there is a significant error that is reflected on the value. This error is attributed to the prevailing temperature conditions that could have caused loss of heat to the surrounding environment. Additionally, draught might have affected the results since it additionally cause the loss of heat (Hunter, 1997).
Lastly, the error might have been caused due to some impurities which could be found in the water implying that the water was not pure during experiment. This implies that the amount of heat required for heating and vaporization might have been lower than the common. However, the valued can be relied on since it is on the range of error that is associated with the latent heat of vaporization in this experiment.
Uncertainties
- The power of the heater was not uncertain since the reading was not obtained directly from the heater
- Uncertainty of beam balance was +/- 1gram
- Uncertainty of time was +/- 0.1 s
- The uncertainty of the latent heat of vaporization is obtained by adding up all the results and finding the average. This could be
Explanations
From the above experience, it is evident that water absorbs a hidden amount of energy so that it can vaporize and change its state of matter from liquid to steam. However, this heat is not visible since thermometer does not show difference temperature. This scenario explains why the steam is a better heating medium that the boiling water.
In this case, steam absorbs latent heat of vaporization enabling it to have more heat than the boiling water. According to my experiment, 1 gram of steam could have additional 2273 joules of heat more than boiling water which is at the same temperature. This implies that it produce more heat than the boiling water, in the same light, when the steam cools down it releases this heat which is absorbed by the object which is being heated by the medium. On the other hand, boiling water would only have the heat that enabled it to reach 100 degree Celsius.
The same explanation can be used to explain why I feel cold when I am sweating during a hot day. In this case, the sweat contains water that is the main component of sweat from the body. On a hot day, high temperature make the water on the skin to evaporate to the atmosphere. As the water evaporates, it absorbs the latent heat of vaporization from the body. This absorption of heat form the body makes the skin to feel cold for a moment. Consequently, the latent heat of vaporization becomes crucial when cooling object. In fact, the cooling systems that use water use the same principle of latent heat of vaporization.
In this case, water gets into contact with the hot metals that make the cooling system. Water is heated up to 100 degree Celsius leading where it vaporizes. During vaporization, it absorbs the latent heat of vaporization according to the mass of water that is used for cooling. This heat reduces the amount of heat which was originally in the system resulting to cooler temperatures than the ones which were originally in the machine.
Conclusion
According to this experiment, the latent heat of vaporization is estimated to be 2073J/ Kg which is roughly accurate as compared to the theoretical values that is 2260J/Kg. it shows that water absorbs hidden amount of heat that is not detectable on a thermometer since it does not cause change in temperature. This heat makes steam a better heating medium than boiling water where steaming is one of the best cooking methods. It, also, explains why people feel cold when sweat evaporates from the skin on a hot day. Additionally, the concept explains the ability of water to act as a cooling liquid in machine since it absorbs the latent heat of vaporization causing reduction in temperature (Wilson & Hall, 2005).
References
Bellium, A, Specific Latent Heat of Vaporization, Home Page.
Hunter, C, 1997, Latent heat, Winnipeg, Man, Nuage Editions.
Lefrois, R, 1979. Active heat exchange system development for latent heat thermal energy storage, Washington, Dept. of Energy, Office of Energy Technology, Division of Energy Storage.
Kent, W,. Heat of Vaporization, Mr. Kent’s Physics Regents Help and AP Physics Exam Review Pages.
Wilson, J, & Hall, 2005, Physics laboratory experiments (6th ed.), Boston, Houghton Mifflin.