Aim
This experiment is aimed at utilizing our basic understanding concerning reactions of alcohol for the elucidation of a more complex reaction. The second goal of the experiment is to change the arrangement of a vicinal diol by shifting a methyl group to make a ketone.
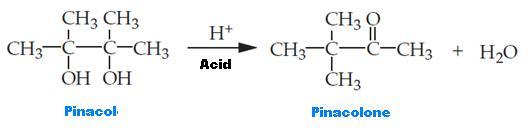
Synthesis equation for the reaction
Physical properties
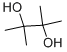
Pinacol
Molar mass: 118.17 g/mol
Melting point: 40-43 °C
Boiling point: 171-172 °C
Density: 0.967 g/mL
Hazards: Irritant, flammable.
Water
Molar mass: 18.01 g/mol
Melting point: 0°C
Boiling point: 100 °C
Density: 0.997 g/mL
Sulfuric acid
Molar mass: 98.08g/mol
Melting point: 10°C
Boiling point: 290 °C
Density: 1.840g/mL
Hazards: Toxic, flammable, corrosive, and irritant.
Sodium chloride
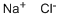
Molar mass: 58.44g/mol
Melting point: 801°C
Boiling point: 1413 °C
Density: 2.17g/mL
Hazards: Irritant.
Sodium sulfate
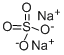
Molar mass: 142.04g/mol
Melting point: 884°C
Boiling point: 1700 °C
Density: 2.68g/mL
Hazards: Irritant.
Pinacolone
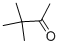
Molar mass: 100.16g/mol
Melting point: -52.5 °C
Boiling point: 106°C
Density: 0.801g/mL
Hazards: Harmful, flammable, and irritant.
For the 2,4-DNP test:
2,4-dinitrophenylhydrazine
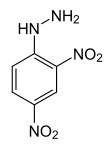
(2,4-DNP):
Molar mass: 198.14g/mol
Melting point: 197-200°C
Density: 0.843g/ml
Hazards: Flammable, harmful
Steps for Pinacol Rearrangement Process
Pinnacol (3.0 g) and deionized water (30 ml) were put in a 50 ml round-bottom flask. A mixture of the two materials was then put in a heating mantle where it was lightly heated up to a point where it dissolved. Tap water was run on the flask containing the mixture for cooling them to room temperature and then 1 to 2 boiling chips were introduced. Concentrated Sulfuric acid (0.5) ml was then carefully added to the mixture bit by bit and in a spiraling motion. The flask was cooled using tap water when it appeared extremely hot. After swirling the mixture for ten minutes, the flask was linked to an uncomplicated distillation apparatus, where the distillate was collected in a round bottom flask (25mL). Once the vapor temperature hit 100 degrees, the distillation process was terminated. Saturated sodium chloride (5mL) was put into the product of the distillation, and then the two were mixed thoroughly. A transfer pipette was employed to get rid of the watery layer. The organic layer was put on anhydrous sodium sulfate to dry up, after which the dried liquid was then decanted, and the sediment-free liquid collected in a clean, dry, pre-weighed, and tightly capped sample vial. The weight of the liquid was measured, followed by the calculation of the percentage of the yield of the product. After the calculation, the infrared and the 2,4-Dinitrophenol were run on the product. The product was capped and put in the laboratory drawer up to the following laboratory session when its nuclear magnetic resonance was performed.
Calculation of theoretical and percent yield
Theoretical yield
- Mass of Pinnacol = 3.0 g
- Moles of Pinnacol = 3.0g/ 118.17 g/mol= 0.02538 mol
- Theoretical yield = (0.02538mol*100.16g/mol)/ 1 mol of pinacolone= 2.542g
Percent yield
- Empty sample vial = 36.3g
- Sample vial + content = 38.02g
- Mass of the product = 1.72g
- Percent yield = (Actual/theoretical) *100= (1.72g/2.542g)*100= 67.66%
Summary of results
Pinacolone, the product that was produced after the pinacol was rearranged, weighed 1.72g while after calculation, the theoretical yield was found to be 2.542g. Besides, the pinacolone product was shown to have a percentage yield of 67.66 percent. This average pinacolone production is attributed to various reasons. For instance, moderate production could be because the pinacol compound was not entirely rearranged into pinacolone; thus the remaining became the reactant. However, the infrared spectrum indicates different results. The spectrum of the product does not indicate any peak about 3500 to 3600 cm-1 ((-OH) stretch), meaning no pinacol remained unchanged after the rearrangement. Furthermore, the moderate percent yield could be due to evaporation of some pinacol compound while being distilled because of high temperature which decreased the pinacol molecule number that was meant for rearrangement into pinacolone. Lastly, other various factors that were difficult to control could have been the reason for the product’s average yield.
Analysis
The rearrangement of pinacol is treated as a dehydration process that takes place alongside various 1,2-diols (glycols). The name of the reaction is drawn from pinacol, the original material, which changes to pinacolone after its rearrangement. The rearrangement permits the production of a carbonyl group from a diol through the process of an SN1 reaction.
Pinacol rearrangement process used acid as a catalyst to speed up the reaction because alcohols are recognized as bad leaving groups. The concentrated Sulfuric acid was utilized in the process as a catalyst for the addition of a proton (H+) to one of the groups of alcohol to produce an excellent leaving group. The reaction of the rearrangement of pinacol is considered exothermic. Tap water was used in place of an ice bath to cool down the round-bottom flask without the occurrence of recrystallization. When the oxygen is protonated in the hydroxyl group, it makes an electron pair to burst into positively charged hydrogen ion and enables the formation of a new oxygen-hydrogen bond. As a result, alcohol is converted into oxonium species, an excellent leaving group.
When water is lost, stable tertiary carbonation takes place. A methyl shift could be used to change the somewhat stable tertiary carbocation to a more stable one. The increased stability occurs as a result of putting the positive charge near the location of the second (-OH) group on the carbon atom. The electrons of oxygen that do not bond with the hydroxyl group assist in enhancing stability on the positive charge via resonance. The product (pinacolone) reacts after a proton from a cation that is stabilized through resonance is removed.
Pinacolone, the product, is different from pinacol, the original material, due to the presence of a carbonyl group in the former. The pinacol rearrangement reaction also produces water, which is immiscible with pinacolone. An uncomplicated distillation apparatus is used to distill pinacolone and water together, the products of the reaction. Concerning Raoult’s law, water and pinacolone are distilled together below their respective boiling points. Water boils and turns into vapor at 100 °C. Therefore, distillation should not continue beyond 100 degrees, the boiling point of water, since it is certain that sufficient co-distillation of pinacolone with water has taken place. Furthermore, if the mixture is heated more, that is, beyond 100 °C, an unwanted result will take place; the distillation of the unchanged pinacol will occur. The aim of adding sodium chloride to the distillate was to eliminate extra water, all inorganic constituents that were impairing the purity of the mixture, and for enhanced separation of the water layer and the pinacolone layer.
The densities of the two products are distinct, and two layers should be formed following the distillation. The top layer consists of organic pinacolone since its quantity of mass per unit volume is less (0.801 g/mL) when compared to the density of the water layer (0.997 g/mL). Water must be separated with pinacolone to prevent its interference with the spectra analysis of the infrared and the nuclear magnetic resonance. The purpose of adding anhydrous sodium sulfate to the organic layer was to ensure that all water molecules are eliminated from the organic layer.
Three forms of assessments were utilized during the reaction to the successful production of pinacolone. The infrared spectrum emphasizes this reaction and demonstrates the conversion of pinacol to pinacolone. The spectrum of pinacol has a (-OH) stretch of approximately 3500-3600 cm-1; a great peak is noticeable from the pinacol’s infrared, indicating the presence of the hydroxyl groups. Nevertheless, (-OH) stretch is shown by the pinacolone spectrum stretch and instead indicates a carbonyl (C=O) approximately 1700 cm-1; that is an indication of ketone synthesis as a result of pinacol rearrangement.
Two peaks are noticeable from pinacolone’s nuclear magnetic resonance, an indication of the presence of two forms of protons in the final products; the protons on the methyl groups and the tert-butyl attached protons. The peak representing the proton type on the tert-butyl group is bigger as compared to the other one on the methyl groups. The difference in the sizes of the peaks is due to the higher number of protons on the tert-butyl than the number of hydrogen on the methyl group (nine protons and three hydrogens, respectively). The two peaks are singlet since there are no protons on the carbonyl group adjacent to the methyl protons. Additionally, the carbon on which the tert-butyl group protons are attached does not have protons on it. Pinnacle’s nuclear magnetic resonance also has the same features as pinacolone’s such as split, peak numbers, and dissimilar magnitudes of the peaks. The only thing that differentiates the two spectrums is the left-shifted peak on the pinacol spectrum’s 2.1 ppm. The concentration and shift show down-shielding of the pinacol’s protons which are connected directly to oxygens. The spectrum of the product does not have any shift, a sign of the production of pinacolone.
The reason why the 2,4-DNP test was done was to indicate that the production of pinacolone occurred after the rearrangement of pinacolone. An orange solid formed as several product’s drops were added to the 2,4-DNP reagent. The solid formation shows that ketone has a double bond between carbon and oxygen. A prompt reaction between 2,4-DNP and aldehydes/ ketones occurs through the condensation reaction. The terminal amino group contained in 2,4-DNP has a single pair of electrons, making it a good nucleophile. An attack on pinacolone’s electrophilic carbonyl group by the nucleophilic 2,4-DNP leads to the formation of an orange precipitate known as 2,4-dinitrophenylhydrazone.