Definitions
- Cancer is a result of abnormal cell division, growth, and invasion of other tissues.
- Neoplasm is a new growth that results in tumor formation.
- Tumors can be benign and malignant.
Before diving into the field of cancer biology, it is essential to review some important definitions and concepts. Firstly, cancer results from abnormal cell division, growth, and invasion of other tissues (Huether & McCance, 2016). The second definition is the neoplasm, a new growth that results in tumor formation (Huether & McCance, 2016). Thirdly, tumors are classified into benign and malignant types based on specific characteristics.
Tumor Types
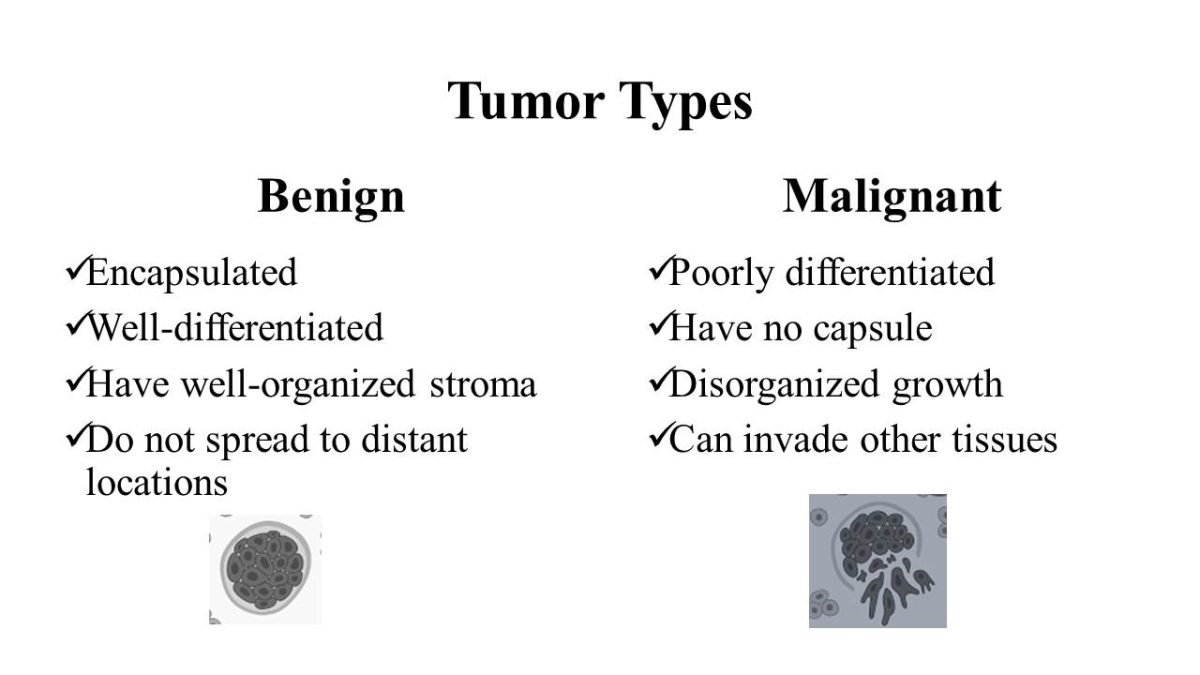
Benign
- Encapsulated;
- Well-differentiated;
- Have well-organized stroma;
- Do not spread to distant locations.
Malignant
- Poorly differentiated;
- Have no capsule;
- Disorganized growth;
- Can invade other tissues.
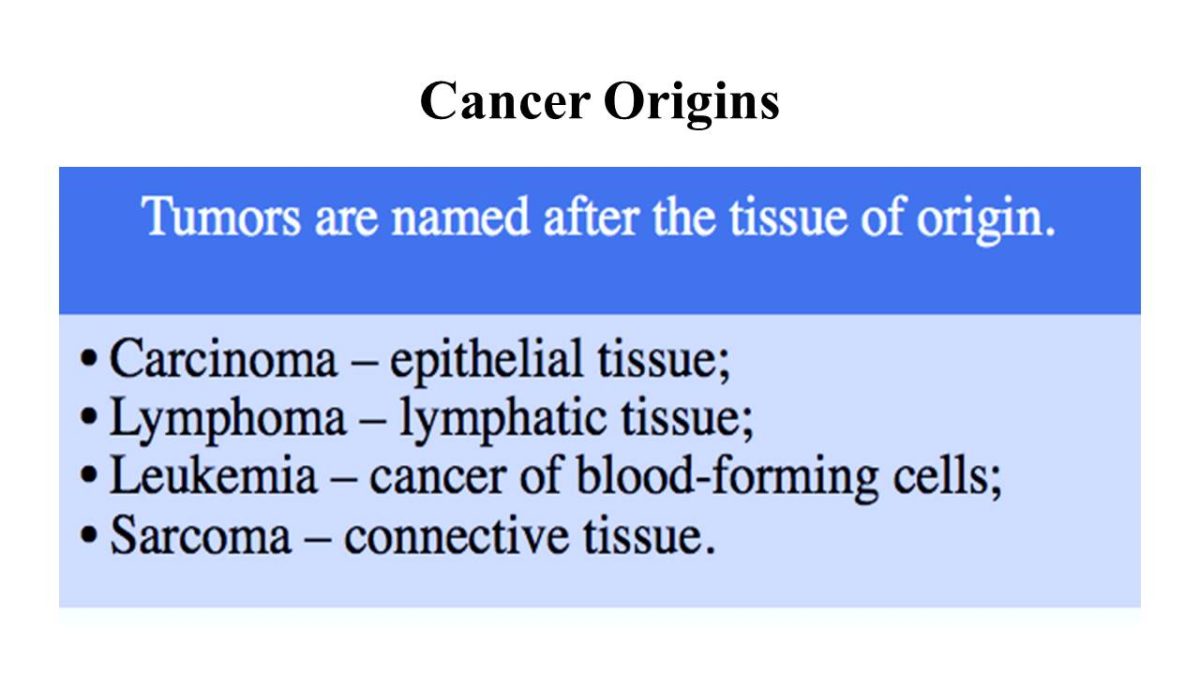
Cancer Origins
Tumors are named after the tissue from which they originated. For example, carcinomas arise from epithelial tissue, lymphomas are cancers of lymphatic tissue, leukemias are cancers of blood-forming cells, and sarcomas come from connective tissue.
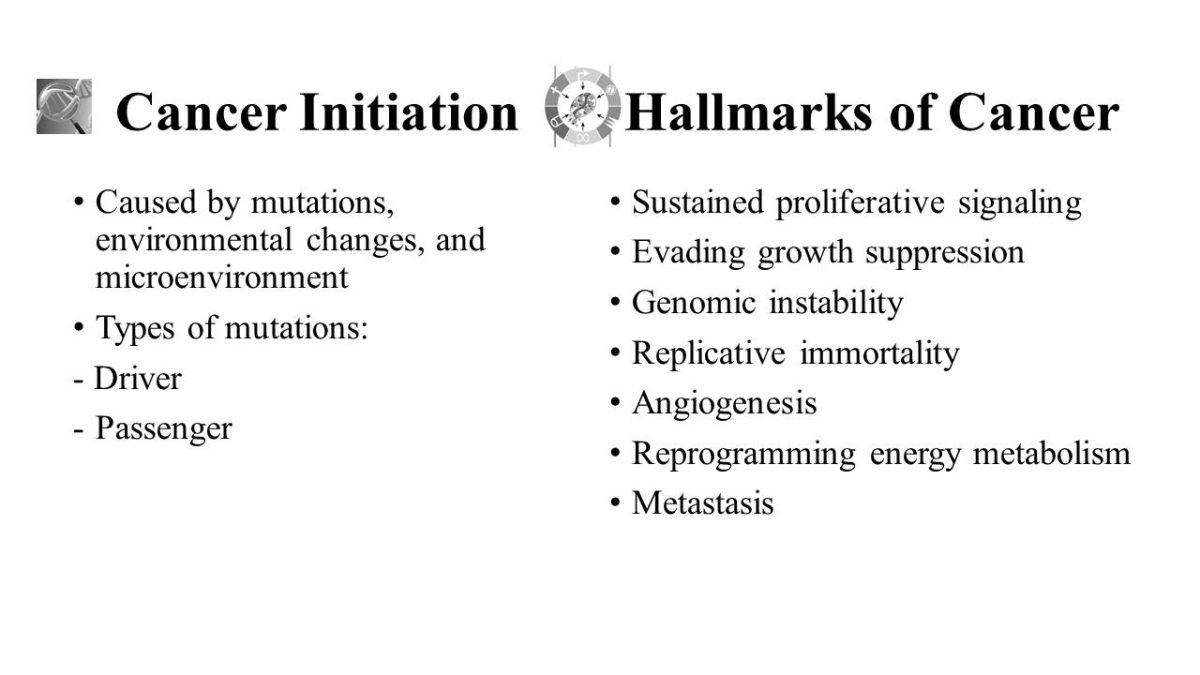
Cancer Initiation
- Caused by mutations, environmental changes, and microenvironment.
- Types of mutations:
- Driver;
- Passenger.
Hallmarks of Cancer
- Sustained proliferative signaling;
- Evading growth suppression;
- Genomic instability;
- Replicative immortality;
- Angiogenesis;
- Reprogramming energy metabolism;
- Metastasis.
Cancers are caused by mutations, environmental changes, and microenvironments.
Two types of mutations are known: driver and passenger.
Cancer is known for its specific features, called hallmarks of cancer. They include sustained proliferative signaling, evading growth suppression, genomic instability, replicative immortality, induction of angiogenesis, reprogramming energy metabolism, and metastasis.
Clinical Manifestation, Diagnosis, and Treatment
- Clinical presentation: Cachexia, fatigue, and pain (at late stages).
- Diagnosis: Imaging, blood tests, and biopsy.
- Classification: TNM staging.
- Treatment: surgery, chemotherapy, radiation therapy, or combined.
Although symptoms vary in different cancers, cachexia, fatigue, and pain at late stages are the most common presentation. Cancer is diagnosed based on the evaluation of several modalities that include blood tests, imaging, and biopsy. Oncologists use TNM staging to classify tumors based on size, lymph node involvement, and metastasis. Various treatment methods were developed for different cancer types, including surgery, chemotherapy, and radiation therapy.
Reference
Huether, S. E., & McCance, K. L. (2016). Understanding Pathophysiology. Elsevier Health Sciences.