The harmful elements in tobacco smoke enter the user’s nervous system, heart, and vital organs within a few seconds of the initial inhalation, affecting each part of the body. However, it is becoming more and more popular to inhale vaporized e-liquid, which is generally considered safer than conventional tobacco products. A propylene glycerol mixture with different nicotine and flavoring percentages is what makes up an electronic cigarette (Stratton et al., 2018). The two most harmful components of tobacco smoke, tar and carbon monoxide, are neither produced using e-cigarettes. Although e-cigarettes are subject to strict safety and quality regulations, they pose certain risks because they are only slightly safer than cigarettes (Uchiyama et al., 2020). This research paper analyses the working principle of microwave plasma atomic emission spectroscopy and microwave accelerated reaction systems. The uses of glycerin, propylene glycol, and nicotine in e-liquid are also evaluated in this paper.
Microwave Plasma Atomic Emission Spectroscopy (MP-AES)
By analyzing a sample’s electromagnetic spectrum or mass spectrum, atomic spectroscopy refers to various analytical methods employed to ascertain the sample’s fundamental makeup. A nuclear emission method is microwave plasma atomic emission spectroscopy (Balaram, 2020). It uses the feature that when an atom of a particular element is stimulated, it releases light in a distinctive range of wavelengths as it rebounds to the initial state (Jung et al., 2019). The inductively coupled argon plasma (ICP) and the microwave plasma (MP), both higher temperature generators and hence suitable stimulation sources for atomic emission spectroscopy, are transmitters of nuclear emission. Temperature readings in the nitrogen-fueled microwave plasma approach 5,000 K (Vudagandla et al., 2017). Atomic emission is substantial at these levels, resulting in suitable identification ranges and linear dynamic spectrum for most components.
As a less expensive and safer option than Flame Atomic Absorption Spectrometry (FAAS) for multi-element evaluation of organic materials, Microwave Plasma Atomic Emission Spectrometry is gaining popularity. The existence of a FAAS flame is a hazard for labs using organic solvents and necessitates regular surveillance (Ozbek, 2018). MP-AES utilizes magnetically linked microwave radiation to create a stable and reliable plasma that may be used to detect critical elements in organic solvents directly (Münzel et al., 2020). The benefits of MP-AES include simplicity of use, excellent performance for challenging organic materials, and a safe approach with minimal operating costs (Qudus et al., 2021). Individual element assessment and the usage of combustible gases like C2H2 and oxidizing gas N2O for certain elements are drawbacks of the procedure.
Microwave Accelerated Reaction System (MARS)
An invention for solvent recovery of environmental contaminants from solid matrices is the Microwave-Accelerated Reaction System (MARS). It is the procedure of separating chemicals from the specimen to the solvent after heating solid sample-solvent combinations using microwave radiation in a closed system (Bizzi et al., 2017). The MARS is equipped to perform solvent separation for organic assessment and acid digesting for inorganic evaluation. MARS is made for usage in laboratories and may be used to extract, dissolve, hydrolyze, or dry a variety of organic compounds (Chew et al., 2019). It is a technology for quick sample preparations for many different analytical techniques. The extraction is carried out in a closed vessel microwave heating technology with temperature and pressure controls.
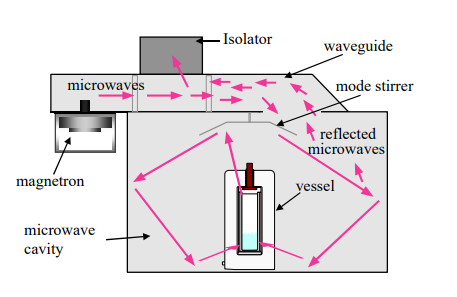
The illustration below shows the microwave system’s main elements, including the magnetron, mode stirrer, isolator, cavity, and waveguide. A microwave powering system on the MARS has a tunable output range of 0 to 1500 watts, plus or minus 5% (Kumar et al., 2020). The magnetron produces microwave radiation, which travels via the waveguide and is injected into the pit. The mode stirrer disperses the energy in different directions, and the hole serves as a confinement container until the material load inside the cavity consumes the power (Nomanbhay & Ong, 2017). The magnetron is shielded from radiation that is not captured by the sampling load by the isolator. Energy may go through the transformer to the pits with the help of the isolator, but it cannot return from the hole to the magnetron.
Glycerin in E-liquid
Most e-liquids are manufactured with a base liquid such as vegetable glycerin, flavorings, and nicotine. According to Akshay (2020), the Food and Drug Association in the United States recognizes flavorings as acceptable for oral intake, sure of the components, such as 2,3-Pentanedione, acetoin, and cinnamaldehyde, are known to inflict irreparable lung damage. Although these substances are acceptable for use in food and beverages, they harm the respiratory system when inhaled (Ind, 2020). Vegetable glycerin, commonly known as glycerol, is an unscented, delightful solution that is colorless or yellowish and naturally occurs in certain living things. Many businesses, including the pharmaceutical and aesthetic sectors, use glycerin (Gotts et al., 2019). Vegetable glycerin can moisten the skin and ease constipation, among other advantages, although these advantages depend on how it is administered.
Sugar alcohol generated from plants, animals, or petroleum is known as glycerin. The kind derived from plant oils is called vegetable glycerin (Soogan et al., 2018). It is believed to have been found by accident more than 200 years ago when lead monoxide and olive oil were heated together. However, it was not until the mid-1900s, when it was initially utilized to produce dynamite, that it started to gain commercial and industrial significance (Woodall et al., 2020). Vegetable glycerin is made by pressing or combining a concentrated alkali, such as lye, with triglyceride-rich vegetable oils, like palm, soy, and coconut fats. This enables the glycerin to separate from the fatty compounds and combine with water to create an unscented, syrup-like liquid with a delicious flavor (Chun et al., 2017). Inflammation of the respiratory system can result from prolonged use of vaping or e-cigarettes, which contain vegetable glycerin. Consuming more glycerin in e-cigarettes is harmful and fatal to the human body.
Propylene Glycol in E-liquid
Organic chemical propylene glycol (PG) is non-toxic and safe for human ingestion. Years ago, cereal, cheese, soft drinks, and ice cream were a few of the commercially accessible foods that contained propylene glycol as a food ingredient (Cotta et al., 2017). Based on e-liquids, propylene glycol gives e-cigarettes their throat sensation and vapor clouds. To keep the components of the e-liquid blended, propylene glycol, a thin, flavorless liquid, is utilized in e-cigarettes (Harvanko et al., 2019). However, propylene glycol levels rise in the blood of e-cigarette users who smoke twice daily for a month, which is harmful (Li et al., 2020). Propylene glycol creates smaller vapor clouds than vegetable glycerin because of its lower density.
When Charles-Adolphe Wurtz originally described propylene glycol in 1859, it was first thought that it would be used in medicinal formulations. It was suggested to swap out ethylene glycol with another substance to act as a solvent and delivery system for a bismuth substance employed to combat syphilis (Martinelli et al., 2019). Comparing propylene glycol to ethylene glycol, which can potentially have adverse effects and even fatal results, short- and long-term pharmacological tests have demonstrated that PG has a minimal side effect when employed as solvents in food and medicines (McGowan et al., 2018). It has a strong affinity for water and readily dissolves in substances including water, ether, glycerol, chloroform, ethyl and methyl alcohols, and ethyl acetate (Smith et al., 2020). Overall, propylene glycol possesses all of ethylene glycol’s great solvent qualities.
Nicotine in E-liquid
A nicotine-containing vapor is what electronic cigarettes are made to offer to the consumer. Commercially accessible e-liquids range from having little to a lot of nicotine. The chemical, which accounts for around 95% of the alkaloid’s composition of combustion in traditional cigarettes and 1% of the volume of cigarette tobacco, is the most prevalent tobacco alkaloid (Hajek et al., 2019). Although some e-liquids are nicotine-free, most are, and e-cigarette nicotine levels can vary (Tiwari et al., 2020). The amount of nicotine present in e-cigarette discharges is a significant factor in determining systemic sensitivity to nicotine and probably has a direct bearing on the misuse potential of e-cigarettes (Bhatt et al., 2020). It is anticipated that the device attributes that change the aerosol’s nicotine content will similarly change how easily e-cigarettes may be abused.
Most e-cigarettes contain nicotine, which is very addictive. The brain and body of e-cigarette users become used to nicotine over time, so once they stop vaping, they may experience nicotine withdrawal problems (Marques et al., 2021). It is challenging to stop using nicotine because of physical and psychological issues. Nicotine releases dopamine in the same brain parts where other addictive substances act (McGrath-Morrow et al., 2020). It brings about changes in mood that enable the individual to feel pleasant momentarily. Smoke is particularly addicting since it takes only 20 seconds for nicotine to reach the brain after inhalation (Picciotto & Kenny, 2020). Pulse rate, cardiac muscle oxygen absorption level, and ventricular pulse pressure all rise with nicotine addiction, causing various respiratory diseases. Nicotine concentrations in the central nervous system decrease after a smoker quits. This alteration sets off the mechanisms that lead to the pattern of desires and impulses that sustains addiction.
References
Akshay, Y., Shrinivas, M., & Chandrakant, M. (2020). Comparative study of conventional and microwave assisted synthesis of some organic reactions.Asian Journal of Pharmaceutical Research, 10(3), 217-220. Web.
Balaram, V. (2020). Microwave plasma atomic emission spectrometry (MP-AES) and its applications -A critical review.Microchemical Journal, 159(42), 201-212. Web.
Bhatt, J. M., Ramphul, M., & Bush, A. (2020). An update on controversies in e-cigarettes.Journal of Paediatric Respiratory Reviews, 36(4), 231-239. Web.
Bizzi, C. A., Pedrotti, M. F., Silva, J. S., Barin, J. S., Nobrega, J. A., & Flores, E. M. M. (2017). Microwave-assisted digestion methods: Towards greener approaches for plasma-based analytical techniques. Journal of Analytical Spectrometry, 32(2), 1448-1466. Web.
Chew, K. W., Chia, S. R., Lee, S. Y., Zhu, L., & Show, P. L. (2019). Enhanced microalgal protein extraction and purification using sustainable microwave-assisted multiphase partitioning technique.Chemical Engineering Journal, 367(1), 1-8. Web.
Chun, L. F., Moazed, F., Calfee C. S., Matthay, M. A., & Gotts, J. E. (2017). Pulmonary toxicity of e-cigarettes.American Journal of Physiology, 313(2), 193-206. Web.
Cotta, K. I., Stephen, C. D., & Mohammad, N. U. (2017). A review on the safety of inhalation of propylene glycol in e-cigarettes.Global Journal of Pharmacy and Pharmaceutical Science, 2(2), 38-46. Web.
Gotts, J. E., Jordt, S., McConnell, R., & Tarran, R. (2019). What are the respiratory effects of e-cigarettes?Journal of BMJ, 2(3), 152-167. Web.
Hajek, P., Phillips-Waller, A., Przulji, D., Pesola, F., Smith, K. M., Bisal, P. N., Li, J., Parrott, S., & Sasieni, P. (2019). A randomized trial of e-cigarettes versus nicotine replacement therapy.The New England Journal of Medicine, 23(21), 234-256. Web.
Harvanko, A., Kryscio, R., Martin, C., & Kelly, T. (2019). Stimulus effects of propylene glycol and vegetable glycerin in electronic cigarette liquids.Drug and Alcohol Dependence, 194(1), 326-329. Web.
Ind, P. W. (2020). E-cigarette or vaping product use-associated lung injury.British Journal of Hospital Medicine, 81(4), 175-181. Web.
Jung, M. Y., Kang, J. H., Choi, Y. S., Lee, D. Y., Lee, J. Y., & Park, J. S. (2019). Analytical features of microwave plasma-atomic emission spectrometry (MP-AES) for the quantitation of manganese (Mn) in wild grape (Vitis coignetiae) red wines: Comparison with inductively coupled plasma-optical emission spectrometry (ICP-OES).Food Chemistry, 274(15), 20-25. Web.
Kumar, A., Kuang, Y., Liang, Z., & Sun, X. (2020). Microwave chemistry, recent advancements, and eco-friendly microwave-assisted synthesis of nanoarchitectures and their application: A review.Materials Today Nano, 11(4), 42-51. Web.
Li, L., Lee, E. S., Nguyen, C., & Zhu, Y. (2020) Effects of propylene glycol, vegetable glycerin, and nicotine on emissions and dynamics of electronic cigarette aerosols. Aerosol Science and Technology, 54(11), 1270-1281.
Marques, P., Piqueras, L., & Sanz, M. (2021). An updated overview of e-cigarette impact on human health.Respiratory Research, 22(151), 162-169. Web.
Martinelli, T., Candel, M. J., Vries, H., Talhout, R., Knapen, V., Schayck, C. P., & Nagelhout, G. E. (2019). Exploring the gateway hypothesis of e-cigarettes and tobacco: a prospective replication study among adolescents in the Netherlands and Flanders. Web.
McGowan, M. A., Scheman, A., & Jacob, S. (2018). Propylene glycol in contact dermatitis: A systematic review. Journal of Dermatitis, 29(1), 6-12.
McGrath-Morrow, S. A., Gorzkowski, J., Groner, J. A., Rule, A. M., Wilson, K., Tanski, S. E., Collaco, J. M., & Klein, J. D. (2020). The effects of nicotine on development.American Academy of Pediatrics, 145(3), 13-46. Web.
Münzel, T., Hahad, O., Kuntic, M., Keaney, J. F., Deanfield, J. E., & Daiber, A. (2020). Effects of tobacco cigarettes, e-cigarettes, and waterpipe smoking on endothelial function and clinical outcomes.European Heart Journal, 41(41), 4057-4070. Web.
Nomanbhay, S., & Ong, M. Y. (2017). A review of microwave-assisted reactions for biodesel production.Journal of Bioengineering, 4(2), 57. Web.
Ozbek, N. (2018). Elemental analysis of henna samples by MP-AES. Journal of the Turkish Chemical Society, 5(2), 857-868. Web.
Picciotto, M. R., & Kenny, P. J. (2020). Mecahnism of nicotine addiction. Cold Spring Harbor Perspectives in Medicine, 10(2), 14-21.
Qudus, H., Purwadi, P., Holilah, I., & Hadi, S. (2021). Analysis of mercury in skin lightening cream by microwave plasma atomic emission spectroscopy (MP-AES).Journal of Molecules, 26(11), 131-145. Web.
Smith, T. T., Heckman, B. W., Wahlquist, A. E., Cummings, M. K., & Carpenter, M. J. (2020). The impact of e-liquid propylene glycol and vegetable glycerin ratio on ratings of subjective effects, reinforcement value, and use in current smokers.Nicotine and Tobacco Research, 22(5), 791-797. Web.
Soogan, L., Henry, N., Hafsa, C., Jill, K., Lisa, D., & Mark, D. (2018). Patch testing to propylene glycol: The mayo clinic experience.Journal of Dermatitis, 29(4), 200-205. Web.
Stratton, K., Kwan, L. Y., & Eaton, D. L. (2018). Public health consequences of e-cigarettes. The National Academies Press.
Tiwari, R. K., Sharma, V., Pandey, R. K., & Shukla, S. S. (2020). Nicotine addiction: Neurobiology and mechanism.Journal of Pharmacopuncture, 23(1), 1-7. Web.
Uchiyama, S., Noguchi, M., Sato, A., Ishitsuka, M., Inaba, Y., & Kunugita, N. (2020). Determination of thermal decomposition products generated from e-cigarettes.ACS Publications, 33(2), 576-583. Web.
Vudagandla, S., Kumar, N. S., Dharmendra, V., Asif, M., Balaram, V., Zhengxu, H., & Zhen, Z. (2017). Determination of boron, phosphorus and molybdenum contnt in biosludge samples by microwave plasma atomic emission spectrometry (MP-AES).Journal of Applied Science, 7(3), 264. Web.
Woodall, M., Kalsi, J. K. K., Schroeder, V., Davis, E., Kenyon, B., Khan, I., & Garnett, J. P. (2020). E-cigarette constituents’ propylene glycol and vegetable glycerin decrease glucose uptake and its metabolism in airway epithelial cells in vitro.American Journal of Physiology, 319(6), 957-967. Web.