Data Collection
Table 1:Recording raw data
Date Processing
Table 2: Processing raw data
In order to determine the concentration of vitamin C in the homogenate, approximately 1 ml of the vitamin C solution was transferred to a test tube. Four test tubes containing 1 ml of vitamin C was setup as replicates. To each test tube, 6 drops of 2% starch solution was added, after which 0.01 M aqueous iodine solution was added to each test tube drop by drop, until the color of the homogenate turned black. The number of drops added to each test tube was taken note of and was used for further analysis. The results of this step of the experiment are presented in Table 1 for data collection.
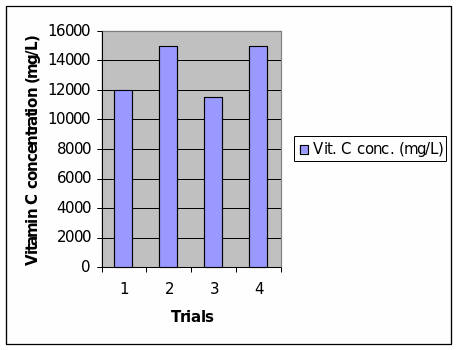
The concentration of vitamin C was calculated by multiplying the number of drops of 0.01 M aqueous iodine solution that was introduced to each of the homogenate in order for the homogenate solution to turn black, by 120. The results of the calculations are presented in Table 2 of data processing. The results are also presented in a column graph, which directly shows that the number of drops of 0.01 M aqueous iodine solution is positively correlated with the concentration of vitamin C in the homogenate. It should be understood that the constant factor in this experiment is the amount of starch in the solution, which is 2% in concentration. Thus, the concentration of vitamin C in each solution influences the number of iodine drops that are needed in order to change the color of the homogenate solution to black. The presence of iodine in a solution triggers the hydrolysis of starch and thus results in the change of color of the solution.
Conclusion and Evaluation
Conclusion
This experiment has allowed us to learn how to prepare a vitamin C solution through the process of homogenization and collecting the homogenate or solution in a beaker. In addition, the experiment also allows us to determine the concentration of vitamin C in a solution through the use of color reactions using an iodine solution of determined concentration. Based on the data we collected, we can show that the amount of vitamin C can be calculated from the number of drops of iodine that was added to the homogenate + starch solution, in order to turn the color into black, which indicates that the starch was hydrolyzed. Based on the data that is presented earlier in Table 2, it shows that more iodine is needed when a vitamin C solution is much more concentrated.
Limitations/Sources of Error
The procedure is straightforward yet there may be steps that could affect our results. Firstly, the size of the drops of iodine may be different, depending on the pressure that is introduced to the pipette. Such discrepancies may be different when another individual adds the iodine to the vitamin C solution. In addition, the rate of dropping may also influence the counting of drops of iodine that is being added to the homogenate solution. If iodine is added at a very fast rate, the counting of iodine drops may be erroneous and if the iodine is added slowly, a better counting of iodine drops will be more precise. There may be fewer errors in the experiment if the dropper used was clean so that there would not be contaminants in the solution. It would have been better if the iodine was introduced in constant volumes in micro-liters using a micropipette so that all drops are precisely measured. For example, the experiment may employ that addition of iodine in 10 micro-liter gradations, instead of simple pipette drops. The use of a micropipette may allow the results to be more accurate in the volume of iodine that is introduced to each test tube. The same method of using a micropipette may also be used for the amount of 2% starch that is added to each vitamin C solution in order to avoid discrepancies in the volume added in the experiment. The purity of the vitamin C solution may also affect the readings of this experiment, wherein there may be impurities that were accidentally included in the vitamin C solution, thus lowering or increasing the amount of iodine that was needed in order to achieve the black color of the solution. Another factor that may affect the experiment is the cleanliness of the glassware, wherein the surface of the test tubes may contain remnants of detergent which may affect the reaction of iodine to the starch present in the vitamin C solution.
Suggestions for further investigations
Other investigations that may be conducted could possibly include fresh strawberries and bottled strawberries, and we can test how temperature affects the vitamin C concentration of the two types of strawberries (fresh versus bottled). From the two sources, we transfer a certain amount of juice to each to three tubes to be kept at room temperature, and we may heat the test tube to 40 degrees once and 60 degrees another time. In addition, we can perform the same procedure on vitamin C tablets versus the chewing vitamin C gum or chewable tablets such as Flintstones, in order to determine vitamin C concentrations in these items. The amount or concentration of vitamin C may also be checked in particular fruits such as grapes, apples and oranges. Even vitamin-supplemented bottled water can be tested for vitamin C content.