The Effect of specific land use practices (sewage treatment, arable farming, livestock farming) on the dispersal of these pollutants to surface waters. The nitrogen cycle has a problem in that human beings are contributing to twice the nitrogen entry into the land-based nitrogen cycle (Vitousek et al, 1997).
The alterations due to human domination have resulted in the increase in global concentrations of the greenhouse gas or nitrous oxide and the formation of photochemical smog (nitric oxide). Land use practices have been faulty to a large extent. Long-term fertility of the soil has been affected due to the loss of essential ingredients calcium and potassium.
The soils and water resources like streams and lakes have become acidified. Policies need to be implemented at the political level for total commitment to prevent water pollution. Management schemes should be need-based and demand-driven.
Decision makers have to understand the requirements of the farmers at grass root levels. Rural settlement problems of agriculture are highlighted as different from urban ones by some ecologists. Water containing the nitrogen has been carried to the estuaries and the coast where it is a major pollutant (Vitousek et al, 1997).
A comprehensive knowledge of the nitrogen distribution to the land and acquatic systems would convey a picture of the nitrogen distributed to the coastal zone (Green et al, 2004).
Simultaneously plants growing in low-nitrogen soils have almost disappeared and the animals and insects which depended on them have also been lost. Plant and animal lives thus have been altered changing the ecosystems. Decline and reduced biodiversity have been seen in the marine life and fisheries (Vitousek, 1997).
Farming
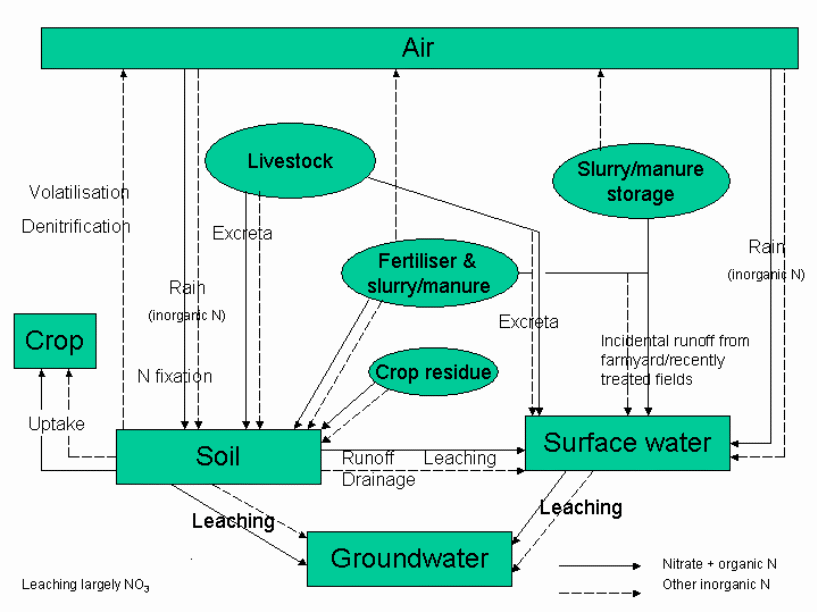
Figure 1 Nitrate pollution originating from agricultural practice (Perry and Gaskell, 2006)
Agriculture accounts for 70% of pollution of soil by nitrates (Sweeten and Auvermann, 2006). Human beings are responsible for increasing fixation, releasing stored nitrogen and also mobilizing nitrogen from the storage pools. The fertilizers contribute the greatest to the human-delivered nitrogen fixation and resultant contribution to the cycle.
Manures and organic nitrogen fertilizers are not included in the contribution of nitrogen fixation by fertilizers: these are involved with mere transfer of previously fixed nitrogen from place to place. There is no new fixation involved with organic fertilizers.
Organic farming adds to the challenges of nitrogen management (Bergstrom, 2005). The substantial increase in nitrogen fertilizer production for the past seventy years has further contributed to the upsets in the nitrogen cycle. The uncontrolled population growth and urbanization have both added to the issue; the increased demand for food would only increase the problem making the future bleak.
Biologically available nitrogen is found only in one-third of the land surface of the earth where crops like soybeans, peas, alfalfa and other leguminous crops grow (Vitousek et al, 1997). The growth of rice too helps in the nitrogen fixation from the atmosphere. It is difficult to measure the amount that is biologically fixed; it could be considered to be 40Tg (teragrams) per year.
Fertilizer fixation comes to about 80Tg per year. Crop residues can replace the nitrogen in rotational arable farming (Wivstad, 2005). Management must include the “mineralisability” of the nitrogen in the crop residue.
Fertilizers may be recommended as a complement to the previous year’s crop residue (Fertilizer recommendations, 2000). Planting earlier or delaying planting may actually cause more pollution as mineralization would continue and cause leaching during the next harvest (Shepherd, 1996).
Burning of fossil fuels like coal and oil releases nitrogen-based trace gases back to the atmosphere from the long-term storage. Vehicles, factories, power plants and combustion processes also make their contribution of about 20Tg (teragrams) to the atmosphere (Vitousek et al, 1997). Humans are also responsible for mobilizing stored nitrogen from the soil organic matter and tree trunks.
About 10Tg per year could be mobilized following the drainage of wetlands. Clearing of land for crops could contribute about 20Tg of nitrogen per year. All the quantity measurements are just approximate values and no one is too sure. Human activities are believed to transfer double the nitrogen from the atmosphere to the land-based nitrogen (Vitousek et al, 1997).
Livestock farming
Livestock contributes to phosphorus pollution by manure production and soil compaction by trampling evidenced by the areas around the water troughs (Sweeten and Auvermann, 2006). The consumption of plants and animals help the farm animals obtain their reactive nitrogen while soil or water provides the reactive nitrogen of plants (Fields, 2004). Livestock farming requires an adequate water supply.
However protection of the water quality and air quality are also part of the livestock farming. In addition nutrients are to be appropriately used for the benefits of the livestock. Mortality disposal is to be in a scientific manner. Energy efficiency and biosecurity have to be specifically attended to (Sweeten and Auvermann, 2006).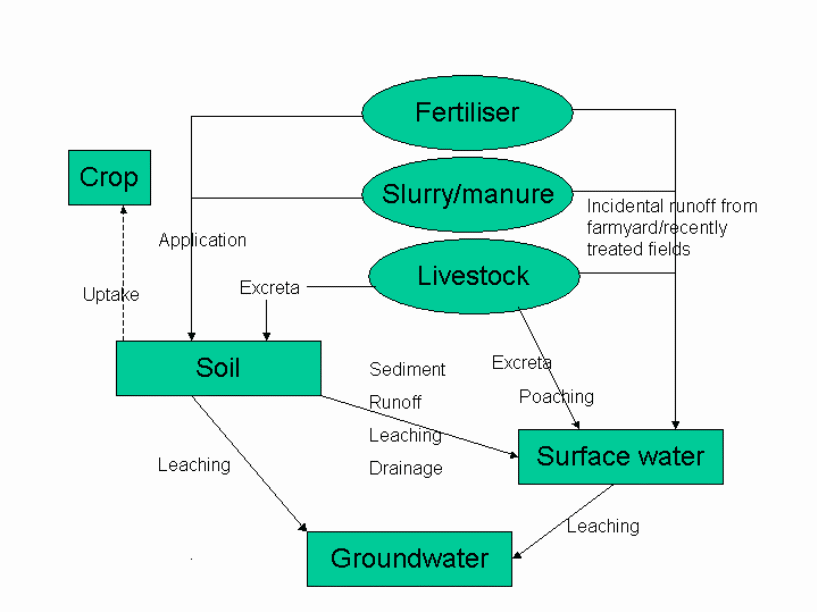
Figure 2 Phosphate pollution originating from agricultural practice (Perry and Gaskell, 2006)
The pollution by nitrates can occur due to the inconsistency of nitrate deposition in the fields. The shed which houses the livestock would hold the slurry especially in the winter while the fields do not have the nitrates. Slurry if deposited on the land due to the shed being over-filled would make the situation worse as there is less plant intake and the runoff is disturbed due to the frozen surface.
Nitrate losses could be minimized by efficient management of the slurry stores (Sweeten and Auvermann, 2006). The figure on page 3 shows how water is contaminated with nitrates and the places where best management practices could work.
Phosphorus excess could occur due to application of slurry and organic manure in the fields (See figure on page 5). The application would be assessed by the nitrogen content without bothering about the phosphorus content. Automatically after application, there is excess of phosphorus.
The N: P ratio of inorganic fertilizers is 8: 1 while cattle manure has the ratio 6: 1 and pig manure has 3:1. In both the livestock manure more phosphorus content is combined (Sweeten and Auvermann, 2006). The bioavailability also varies between the nitrogen and the phosphorus. Phosphorus is mostly bioavailable while the nitrogen is only half bioavailable.
Sewage treatment
Appropriate treatment of sewage before release into the rivers is essential for preventing pollution of water. Treated effluent has its uses: in gardening and for cooling (Pollution, Chapter 5). Planning for sewage treatment plants, crematoriums and low cost sanitation facilities must be the responsibility of the local policy makers and planners. Sewage treatment plants decrease the burden of heavy pollution on the sea waters.
The impacts of the pollutant on water quality and ecosystem function
Land-based nitrogen has produced a vast increase of nitrates in the surface waters. Bodies of water have shown a six-fold to twenty fold increase. The input would have come from watersheds polluted from the atmosphere and fertilizers.
Lakes in Norway and rivers in the US have shown more than double the rise in concentration of nitrates. The rise in the density of population has a direct relationship to the rise in concentration of nitrates. However the total nitrogen may not rise in the same proportion as the nitrates (Vitousek et al, 1997).
Nitrates in surface water have shown an increase in agricultural land. It is difficult to measure the extent of the problem. The nitrates in the surface water may not contribute much to the ground water. Drinking-water pollution with high level of nitrates can cause problems in infants especially (Vitousek et al, 1997).
Conversion of the nitrates to nitrites causes problems when absorbed into the blood. Methemoglobin is formed from hemoglobin. Elevated level of this is methemoglobinemia which causes brain damage or death. It is however rare.
Acidification of lakes and streams are occurring in to two ways. The acid rain, snow, fog and other such climatic issues contribute to the acid deposition in soil and water. This acid deposition is prevented by controlling the atmospheric emissions of sulphur dioxide. However the nitric oxide which forms nitric acid is neglected in the process.
One point to note here is that soils are becoming saturated and cannot neutralize the acid deposition before the surface water flows to the streams (Vitousek et al, 1997). The streams get all the excess nitrates. The winter snowpacks produce another problem. With the melting of snow, there is a sudden rush of nitric-acidic water into the streams. A sudden rise in acid concentration would have its ill-effects on the vegetation and fisheries.
Ecosystems are also rich in phosphorus. When the inorganic nitrogen reaches the freshwater systems, eutrophication and acidification occur (Vitousek et al, 1997). The plant and animal species become less diverse. The fish populations reduce in number and diversity. Control of the sulphur dioxide emissions alone will not control acid rain and its deposition on the ground soil and streams and lakes.
In temperate zone fresh-water lakes phosphorus is responsible for the eutrophication by limiting the activity of the algae and other plants. In temperate zone estuaries and coastal waters it is the nitrogen inputs which control the situation.
Here the nitrogen that flows into the water is minimal. The fixation of the nitrogen by the plankton is also low. At the same time microbes found on the sea floor rapidly release the nitrogen back into the atmosphere (Vitousek et al, 1997). So very little nitrogen is lost.
When surface waters are loaded with nitrogen, there is no mixing of waters of different temperatures. The colder water is below the surface which has warmer water. The bottom layer will be anoxic or hypoxic. However this phenomenon is less seen in freshwaters.
Cost effective measures for mitigating the impacts of the land use practices either at source or in the receiving water
Proper use of land, timely application of the right amount of fertilizers, waiting for the right climate, ensuring proper drainage and the best management practices influence nitrate pollution (Sweeten and Auvermann, 2006; Shepherd and Chambers, 2007). Reducing the production of nitrogen fertilizer could slow down their use and mobility.
Increasing its efficiency is another means of solving the problem. Usually the fertilizer applied will be half lost to the air and water. The agricultural plants do not utilize all of it. This loss can be reduced and the efficiency increased (Vitousek et al, 1997).
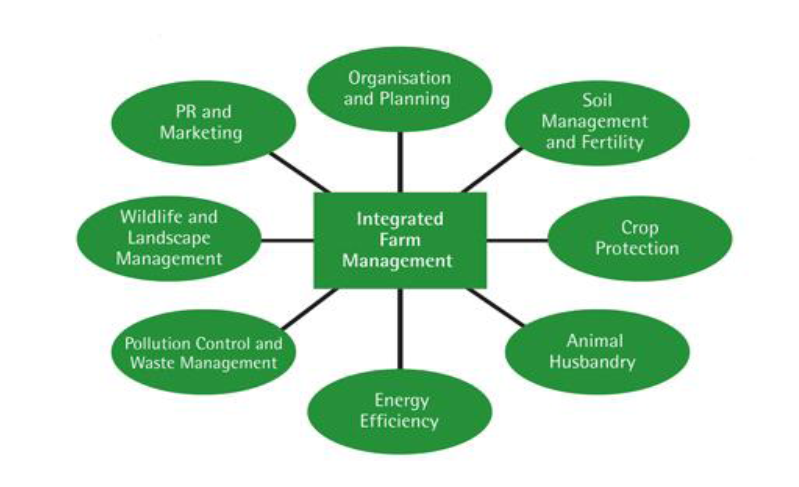
Figure 3 Prevention of Environmental Pollution from Agricultural Activity www.defra.gov.uk/evidence/statistics/foodfarm/…/observatory/…/obs03.
The fertilizer that is wasted has all the nitrogen that causes the pollution. This waste is expensive and produces harmful environmental changes and has to be reduced. The best management practices would decrease the amount of fertilizers used and reduce the losses into air and water. Yields or profits must not be affected.
One method of preventing waste is to mix the fertilizer in irrigation water and allow the plants to receive the nutrition below the surface. Channels of water should not be allowed to run in between the plants in the agricultural land. Riparian forests should not be cleared and wetlands should not be drained so that fertilizer is not wasted (Vitousek et al, 1997).
Excess nitrogen can also be prevented from polluting streams and lakes by restoring wetlands and areas of riparian forests. Emissions from fossil fuels need to be reduced in the coming years. Fuel combustion must be controlled and made more efficient so that airborne by-products are reduced. This is especially significant with the rapid global growth of economy and industry (Vitousek et al, 1997).
In the livestock farming application of slurry or manure is avoided in the winter to prevent runoff. Phosphorus pollution occurs when slurry or manure is applied without regard to the climate, soil type, geography or with inappropriate management. Spreading the slurry in partially dry, cracked soil before a rain is advisable. Slurry application needs to be optimal so that it is done only in favorable conditions.
Slurry storage also must be limited to reduce losses. Application techniques could include injection and incorporation of manure. In the process, nitrate leaching could increase and must be avoided. Precision farming could prevent an excess of phosphorus in the land (Sweeten and Auvermann, 2006).
Non-inversion tillage led to greater phosphorus in the surface when compared to ploughing in a study (Sweeten and Auvermann). However in both techniques drainage water had little phosphorus. Controlling the livestock density, providing access to water courses and targeting the grazing area based on soil conditions help to control the phosphorus levels in the soil (Sweeten and Auvermann).
Sulphur dioxide emissions can be controlled to prevent acidic deposition. Appropriate sewage treatment plants, planned sites for crematoriums and low cost sanitary facilities help in reduction of pollution of air and water.
Best management practices must be redefined (Shepherd and Chambers, 2007). The attempt to manage the nitrogen cycle more biologically than before poses a challenge (Millenium Ecosystem Management, 2005). The figure 3 on page 9 indicates how integrated management of of the environment can prevent pollution.
Several papers have reviewed and listed these practices (Jarvis, 2000). Single practices have been researched with the aim of refining the protocol (SUM, 2005). Pollutants could interact with each other and produce further damage to the environment and water.
Research had indicated an increase in ammonia emissions when slurry was applied in June when previously it was done in October to reduce the leaching of the nitrates (Williams et al, 2006). The slurry had infiltrated less into the soil because of the warm temperature and dry soil.
Different cycles of nutrients also can have “pollution swapping”. Efforts of researchers must focus on increasing the efficiency of nutrients. Protection of soil quality by research of the biological, physical and chemical properties would evolve a new ecosystem protocol and this must be a priority (Stockdale, 2002).
Another strategy is to keep the price of the fertilizer and the product at an optimum. This would reduce over-fertilization. The present problem of over-fertilization to increase the yield needs to be managed. Research may correct the situation (Shepherd and Chambers (2007).
Nitrogen-use efficiency has been found to be much greater in organic arable systems than organic dairy systems. Efficient management of manure production include changes in the diet of the livestosck, the thin spreading of manure over a large piece of land and making the manure more manageable.
Conclusion
Human activities have doubled the nitrogen that enters the land-based nitrogen cycle and causes serious environmental changes. Soils and waters are being acidified producing a change in the flora and fauna. Diversity is being lost or reduced in aquatic and land ecosystems. Human contribution for alterations in the nitrogen cycles has to be controlled to prevent eutrophication and acidification of freshwaters.
The threat to the ecosystems must be prevented. Farms are to initiate best management practices to reduce the load of pollution to the environment, fresh waters and marine waters. Management of the nitrogen cycle at the farm level has many challenges but solution of conflicts should eliminate losses of nitrogen to the environment. Change can be implemented through the right policies.
References
Bergstrom, L., Bowman, B.T., and Sims, J.T. (2005). Definition of sustainable and unsustainable issues in nutrient management of modern agriculture. Soil Use Management Vol. 21:76-81
Fertilizer recommendations for agricultural and horticultural crops (MAFF Reference Book 209).The Stationery Office, London
Figure 3 Prevention of Environmental Pollution from Agricultural Activity www.defra.gov.uk/evidence/statistics/foodfarm/…/observatory/…/obs03.
Fields, S. (2004). Global Nitrogen: Cycling out of control. Environmental Health Perspectives Vol. 112(10): A56- A563 July 2004
Green, P.A., Vorosmarty, C.J., Meybeck, M., Galloway, J.N. Peterson, B.J. and Boyer, E.W. (2004). Pre-industrial and contemporary fluxes of nitrogen through rivers: a global assessment based on typology. Biogeochemistry, Vol. 68:71-105 Kluwer Academic Publishers
Jarvis, S.C. (2000). Progress in studies of nitrate leaching from grassland soils. Soil Use Management Vol. 16: 152-156
Millenium Ecosystem Assessment, Ecosystems and Human Well-being: Synthesis, Island Press, Washington D.C. (2005)
Perry, H. and Gaskill, P. et al. (2006). Agricultural change and environmental observation programme: Quantitative approaches to assessment of farm level changes and implications for the environment. Final Report, October 2006.
Shepherd, M.A., Harrison, R. and Webb, J. (2002) Managing soil organic matter-implications for soil structure on organic farms. Soil Use Management Vol. 18: 284-292
Shepherd, M. and Chambers, B. (2007). Perspective managing nitrogen on the farm: the devil is in the detail. Journal of Sci Food Agric.Vol. 87: 558-568 Society of Chemical Industry
Stockdale, E.A., Shepherd, M.A., Fortune, S. and Cuttle, C.P. (2002). Soil fertility in organic farming systems-fundamentally different? Soil Use Management Vol. 18:301-308
SUM (2005) Nutrient management in sustainable agricultural systems. Soil Use Management, Vol. 29 (1):1-166
Sweeten, J.M., Auvermann, B.W. (2006). What’s ahead in water, air and environmental quality issues affecting the beef feedlot industry: A Southern Great Plains perspective. The John M.Airy Symposium for Animal Agriculture and the Environment. January 2006.
Vitousek, P.M., Aber, J., Howarth , R.W.,Likens, G.E. Matson, P.A. and Schindler, D.W. et al,
(1997)Human alteration of the global nitrogen cycle: causes and consequences. Issue in Ecology, No. 1 Spring 1997. Ecological Society of America, Washington.
Williams, J.R., Sagoo, E., Chambers, B.J., Cross, R.B., Short, J. and Hodgkinson, R.A. (2006). Nitrogen losses after cattle slurry applications to a drained clay soil in Managing Rural diffuse pollution Ed. Gairns,L. Crighton, C. and Jeffrey, B. SEPA, Edinburgh, p. 267-271
Wivstad, M., Dahlin, A.S. and Grant, C. (2005). Perspectives on nutrient management in arable farming systems. Soil Use Management Vol. 21:113-121