Introduction
A Microbial Fuel Cell (MFC) is a gadget capable of transforming chemical energy to electrical form. It utilizes micro-organisms for the required catalytic reactions. Certain types of bacteria such as Shewanellaputrefaciens and Aeromonashydrophila are known to release electrons as part of their metabolic activity. Such bacterium feeds on micro-organisms found in wastewater (Aelterman, Freguia, Keller, Verstraete & Rabaey 2008, p. 408). If this bacterium is attached to a cathode of a controlled electric system such as a microbial electrolysis cell and then exposed to wastewater, the result is an electric current. Since these electron-producing bacteria are consuming micro-organisms in the water, the water’s Biological Oxygen Demand (BOD) levels are consequently reduced. This is a critical discovery when considered comprehensively for exploitation. Technological advancements are inevitable in the present world. Additionally, their establishment, implementation, and embracement should be considered effectually.
Evidently, the use of Microbial Fuel Cells is a recent technology; hence, there is relatively little research in the area. Further, the science behind some of the aspects of bacterial electro chemistry is little understood. The viability of this kind of technology is extensive as it poses a potentially viable alternative to fossil fuel energy. Also, such technology offers a new and potentially low cost method of wastewater treatment.
Importance/ Significance of study
Conventional sewage treatment methods require high energy and are costly. There is a need to find alternative methods that are less costly and use less energy for operations. The use of high rate anaerobic methods has increased in recent years. This is applicable in the treatment of domestic and industrial wastewater and other related refuse. These have significant merits over the conventional treatment methods (Rabaey 2009, pp. 343). The merits are that no oxygen supply is required, and there is minimal sludge in production. Additionally, methane gas is recovered form the operation. The conventional treatment method in small plants usually leave the methane gas go to waste. The gas is lost through the seepage of the reactors used. Thus, conventional waste water treatment methods are not efficient. The concentration of the methane gas lost is likely to be about 16 mg/L. This is a high level of concentration due to high pressure in the reactors used in the conventional treatment plants. This reduces opportunities for energy recovery that may be made (Lefebvre, Uzabiaga, Chang, Kim & Ng 2011, pp. 259).
Furthermore, environmental concerns and the need for energy security give a reason for new sustainable and clean sources of energy. The new energy sources should use no or minimal hydrocarbons. Different types of plants or batteries can be used in the production of energy. One other way of producing electricity can be through the use of fuel cells. The conversion of organic matter can be catalysed by bacteria. This can result into production of electricity. There are bio-fuel cells that add artificial electron shuttles and produce electricity. However, microbial fuel cells do not need addition of electron shuttles. The fuel cells usually convert energy from one form to another. They are not like batteries that store energy. They also produce energy continually as long as they are fed. Moreover, the change of chemical energy to electricity in fuel cells is direct. Fuel is not first converted to mechanical energy as batteries do. Thus, in case MFCs are utilized to treat the concerned waste water, the majority will be able to access affordable and safe energy as well as clean water. Consequently, the gains attained from utilizing MFCs incorporate clean and safe energy. Additionally, the emissions are low they are easy to operate and energy efficiency is high (Rabaey 2009, p. 343).
Project Outline
Microbial Fuel Cells (MFCs) can be used in treatment of waste water and at the same time production of electricity. With the growing concern for the need to find better ways of energy production, the use of the technology will be beneficial to the world. The conventional methods of waste water treatment are inefficient and costly. This topic is of interest as there is a valid reason to find more fuel efficient ways of energy production. The proposal intends to execute a study regarding the viability and applicability of microbial fuel cells in the treatment of waste water as well as generation of electricity.
Abilities
There are required skills to undertake the project. Extensive academic studies have been executed on the topic. There is need to conserve the environment as mandated by this technology. My degree choice has also enabled me obtain a deeper understanding of how microbial fuel cells operate. I have compared it with conventional waste water treatment methods and other sources of fuel. Finally, I am an organized fellow, with excellent organizational and researching skills. These will enable me undertake the research within the stipulated time.
Specific objectives and Justification
The major goal of the project is to determine the viability of microbial fuel cells in treatment of waste water and production of energy. This will enable a massive supply of clean water to a large population, especially the poor, and supply the population with electricity. This is mainly because the microbial fuel cells can be produced cheaply compared to conventional energy production methods. The project is viable as laboratory examinations have produced positive results. In addition, the materials required for carrying out the experiments required are easily available and are affordable.
Assumptions
The project makes an assumption that membrane-less microbial fuel cells can be used to produce high voltage power. In order to verify this, a microbial fuel cell with a membrane separating the anode side and the cathode side will be used. This will be compared with the operation of a microbial fuel cell that has no membrane. The other assumption is that various microbial fuel cells can be joined together to produce high voltage. The assumption is made since single microbial fuel cells produce relatively low energy voltage compared to the conventional. The various microbial fuel cells will be joined to determine whether the energy voltage produced is increased.
The other assumption is that the whole community will see the need to use more fuel-efficient methods of energy production. If the whole community embraces the technology, there will be considerable savings since energy will be produced cheaply. The health status of the community will improve through the use of clean water. The acceptance of the technology will be determined through further researches.
How Bio-Fuel Cells Work
Biocatalysts are used in bio-fuel cells to convert energy from chemical to electrical energy. The device directly converts microbial energy to electricity. The device does this by use of conventional technology in use. The biocatalysts take part in transfer of electrons between fuel substrate and surfaces of electrodes. Evidently, this shows that the concerned micro-organisms are able to enhance the transfer of electrons amidst the fuel substrate and the surfaces surrounding the electrodes. The result is that a current is created in the cell (Lefebvre, Uzabiaga, Chang, Kim & Ng 2011, pp. 259). The transfer of electrons from the microbial cells takes place at low efficiency. This is because the transfer is direct. It is important to note that most microbial cells appear to be dormant electrochemically. Thus, mediators are used to enable transfer of electrons to the electrodes form the microbial cells. These mediators include humic acid, thionine, and methyl-viologen among others. NADH (dihydro-nicotinamide adenine dinucleotide) can be generated from substances that can enable biotransformations that result in activation of the anode compartment of the bio-fuel cells. These substances can include alcohol, amino acids, and lactic acid. The generation of NADH can be catalysed through the use of enzymes. In theory, organic and inorganic compounds oxidized by suitable organism results into fuel. An example is the reaction of glucose and water (Lefebvre, Uzabiaga, Chang, Kim & Ng 2011, pp. 259).
Waste Water Treatment and Energy Production Aspects of Microbial Fuel Cells
Various studies done have indicated that some ion-reducing bacteria can transfer electrons to the electrodes. These bacteria transfer the electrons directly using redox enzymes that are electrochemically active (Logan 2008, pp. 40). The bacteria are mainly of the family Geobacteraceae. These include Pseudomonas species, Shewanella species, Geothrix species and Escherichia coli. One such redox enzyme is cytochromes. These types of microbial cells do not need mediator to enable transfer of electrons. They are known as mediator less Microbial Fuel Cells. They have commercial viability and applicability. This is because the mediators normally used in Bio-fuel cells are costly. Additionally, they can be toxic to various micro-organisms (Logan 2008, pp. 40).
Two electrodes are used in microbial fuel cells. These are cathode and anode, and they are placed in water. They are separated using a proton exchange membrane. It is crucial to agree that most researches done indicate that graphite, carbon cloth, graphite-felt, as well as graphite coated with platinum can be utilized to enhance the reaction. The microbes in the anode side usually oxidize fuel. This generates electrons and protons. The electrons are then transferred to the cathode side. Agreeably, this is executable through an exterior circuit. Conversely, it is notable that protons will be transferable through the well-set membrane. Consequently, it is scientifically evident that the electrons together with protons are consumed at the cathode plane. This results into reduction of oxygen.
There are other organisms that further promote the performance of microbial fuel cells. These are different from micro-organisms that facilitate the transmission of electrons to the anode. A mixture of culture of organisms can generate much electricity that use of one type of culture. This means that the microbial communities at the anode side can perform similar roles to those performed by methanogens in methanogenic aerobic digesters (Jung & Regan 2007, pp. 394). It is important to note that the sole disparity is that micro-organisms capable of transferring electrons to the electrodes can be used in this context. They replace methanogens used in anaerobic digesters. Notably, Adapted Anodophilic Consortia (terminology) is used by numerous researchers to denote these microbial communities. Anodophilic bacteria of different families such as Geobacteraceae, Alteromonadaceae and Aeromonadaceae are capable of transferring electrons to the electrodes. Others are Comamonadaceae, Desulfuromonaceae and Clostridiaceae. It must be noted that the Methanogens are also capable of transferring electrons (Aelterman, Freguia, Keller, Verstraete & Rabaey 2008, pp. 408).
Microbial fuel cells have relatively low power output when compared to other fuel cells. It is, therefore, necessary that they have lower costs. This has to be done if the generation of power using microbial fuel technology is to be used in production of energy (Crabtree 2010, pp. 83). This challenge is to be overcome, and thus this study is justified. There is a valid reason to find a method of enhancing power production using microbial fuel cell. This can be done by tackling the limitations the technology has.
The demerit of using two chambers in the microbial fuel cells is that the cathode solution has to be aerated (Feng, Wang, Logan & Lee 2008, pp. 83). This is to provide oxygen to the cathode. The amount of power that the microbial fuel cells produce can be enhanced by improving the performance of the cathode. An example of improving efficiency can be through the addition of ferricyanide to the cathode side. It is vital to recognize that MFCs whose cathodes are hardly dipped in water can be structured/designed. The cathodes of hydrogen fuel cells are bonded directly to the proton exchange membrane (PEM). This ensures that oxygen can reach and react with the electrode directly. This method can produce electricity from waste water.
Membrane-Less Microbial Fuel Cells
Some of the microbial fuel cells do not have mediators. The membrane in these cells separates the anode and cathode like in other microbial fuel cells. Furthermore, the membrane performs the role of an electrolyte. This means that the membrane acts as an electric insulator. The membrane allows protons to pass through it. However, use of membranes in microbial fuel cells limits the usage and efficiency of the devices in treatment of waste water. The transfer of protons through the membrane is a limiting factor (Wang 2010, pp. 37). This is mainly because there are suspended solids in waste water. Additionally, there are soluble contaminants in waste water treatment processes. The membrane-less microbial fuel cells have been successfully used in production electricity. They are under utilization to enrich microbes. These microbes must be electronically charged in order to convert organic contaminants (existing in waste water) to electricity. This further shows that microbial fuel cells can be used to produce electricity and at the same time be used to clean waste water.
Methods
The project will provide extensive literature on the use of microbial fuel cells. The literature will trace the development of the technology, and the various models of the microbial fuel cells that have been developed. It will further compare the merits that the technology has over the conventional methods of waste water treatment and energy production. Additionally, laboratory models of a large project will be used during experimentations. This will be done so as to enable data collection, which will be employed in determination of the viability of the project in a large scale.
Merits and Limitations
The main limitation of using microbial fuel cell in treatment of waste water is that the cells produce relatively low amount of power. Additionally, the technology is still in its inception state. Fortunately, this problem may be solved through linking of many microbial fuel cells. Notably is possible to enhance the voltage of the MFCs through the linkage of numerous cells.
The costs of the microbial fuel cells can be reduced further by use of electrodes made of ordinary graphite. The microbial fuel cells can also operate without having electron shuttles that are exogenous. The membranes used are also commercially available. These further show the viability of using these cells in the treatment of waste water and production of energy.
Generally, however, there is a need to improve the power density of the cells. It is crucial to note that the nature of MFCs can limit the transfer rates of electrons to the anode. Thus, the microbial fuel cells require optimization. The optimization of the cells should involve inspection of various aspects of the cells. The mode of electron transfer should be appropriate the performance of the cathode has to be checked, and adequate oxygen supply has to be ensured. Additionally, the nature of organic material in the waste water has to be determined, the concentration of bacteria has to be known, and external resistance to the systems must be known too.
Resources and Strategy
The project experiment will need anodes and cathodes made of ordinary graphite. The anode will be placed at the bottom while the cathode will be placed at the top of reactor that is cylindrical in shape. The reactor will be made of polyacrylic. The reactor will have a diameter 10 centimetres. Glass wool together with beads must be installed over the anode. The reactor will have a height of 60 centimetres and the anode and cathode will be 20 centimetre apart. Fuel will then be supplied to the lower part of the anode. Consequently, it is vital to connect the electrodes to a copper cable.
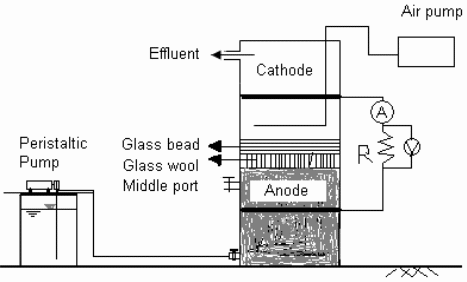
The final setup will appear as shown in the above diagram. Waste water will then be pumped at a rate of 5.011 L/d. Consequently, it is notable that the cathode side of the cell shall aerate at 60 mL/min. This will allow a total reaction on the cathode cable. An artificial sewage (synthetic waste water) with some sucrose acting as carbon source will be used (Feng, Wang, Logan & Lee 2008, pp. 83). All the materials that will be used in this project will be obtained in the laboratory. The laboratory assistants will assist in setting up the cell and in monitoring the performance. The project is straightforward and thus there will be no need for assistance from a professional.
Data to be collected
The project will measure the potential by use of a digital multimeter. The potential will be transformed to power according to P= iV, under which P= power (w) and i= current (A), and V= Voltage. Other data which will be collected or monitored in the project include effluent, pH, and dissolve oxygen. The demand for oxygen in order to make the project successful will be determined.
Timeline
The expected timeline of the experimental part of the project will be three weeks. The key tasks will be gathering of all the necessary materials, setting up of the microbial fuel cell, and collection of data. However, the timeline before actual implementation of the real project will be two months. This is because environmental impact assessment of the project will be carried out. This will focus on the environmental, social, economic and health impact that the project will have on the community.
Project Risk Assessment
One of the risks that the project may run into is to depletion of key resources. Thus, the assistants at the laboratory will have to put in place more of the resources especially the graphite rods and artificial waste water. The other problem is that at the implementation stage, the community may not be quick in adopting the technology. The community will have to be first educated on the benefits of the technology before the actual implementation of the project begins.
Conclusions
Microbial Fuel Cells (MFCs) can be used in the treatment of waste water and production of electricity. The objective of this proposal is to execute a project that will determine the visibility and feasibility of MFCs in the treatment of waste water and production of energy. Additionally, the project will aim at improving the livelihoods of the general population. MFCs can provide efficient means of waste water treatment and energy production. They have merits over conventional methods used currently. Additionally, they are less costly compared to the conventional methods used currently in treatment of waste water and production of energy. The components used in the designing the microbial fuel cells are also easily obtainable. The only limitation that the microbial fuel cells possess is that they have a lower energy output when compared to the conventional methods of waste water treatment and energy production. However, various microbial fuel cells will be linked together to improve the output capacity in the project. Additionally, their commercial visibility can be improved by removal of membrane separating the anode side and the cathode side of the cells. The other limitations that the project will face will be tacked effectively.
List of References
Aelterman, P., Freguia, S., Keller, J., Verstraete, W. & Rabaey, K 2008, “The anode potential regulates bacterial activity in microbial fuel cells”, Applied Microbiology and Biotechnology, vol. 78, no. 3, pp. 409-18.
Crabtree, R 2010. Energy production and storage: inorganic chemical strategies for a warming world, Wiley, Chichester.
Feng, Y., Wang, X., Logan, B & Lee, H 2008, “Brewery wastewater treatment using air-cathode microbial fuel cells”, Applied Microbiology and Biotechnology, vol. 78, no. 5, pp. 873-80.
Jung, S & Regan, J 2007, “Comparison of Anode Bacterial Communities and Performance in Microbial Fuel Cells with Different Electron Donors”, Applied Microbiology and Biotechnology, vol. 77, no. 2, pp. 393-402.
Lefebvre, O., Uzabiaga, A., Chang, I.S., Kim, B. & Ng, H 2011, “Microbial Fuel Cells for Energy Self-sufficient Domestic Wastewater Treatment–A Review and Discussion from Energetic Consideration”, Applied Microbiology and Biotechnology, vol. 89, no. 2, pp. 259-70.
Logan, B 2008. Microbial fuel cells, Wiley-Interscience, Hoboken, NJ.
Rabaey, K 2009. Bioelectrochemical systems: from extracellular electron transfer to biotechnological application, IWA Publishing, London, UK.
Wang, L 2010. Membrane and desalination technologies, Humana, New York, NY.