Background Information
A critical environmental and public health issue is the harm caused to human communities by insects. Mosquitoes, flies, fleas, and ticks bring discomfort to daily life by leaving itchy spots and redness on the skin of individuals. Moreover, many insects, especially in tropical climates, are carriers of infectious diseases transmitted to humans through the bite (DEP, n.d.). Examples of such infections are viral encephalitis, Ebola, malaria, sleeping sickness, and other pathological conditions associated with the development of pathogens within the human body. Thus, a legitimate need is created for the use of defenses to inhibit the transmission of infections and rid the community of discomfort from insects.
One strategy for such protection is prophylactic measures to prevent the spread of vector-borne infections. The Deet Education Program (DEP) tells users about the significant risks and threats of being outdoors and insect repellents (DEP, n.d.) for free. DEP also talks about the main component of chemical insect protection, namely DEET. DEET is historically proven and has been used as a commercial protective agent for over sixty years (DEP, n.d.). In terms of molecular structure, the study material reports that DEET stands for N, N-diethyl-m-toluamide, which means this substance is classified as an amide (Figure 1). Amides, strictly speaking, are derivatives of carboxylic acids whose functional group is modified by the addition of an amino group; from Figure 1, we can see that DEET contains a benzene ring.
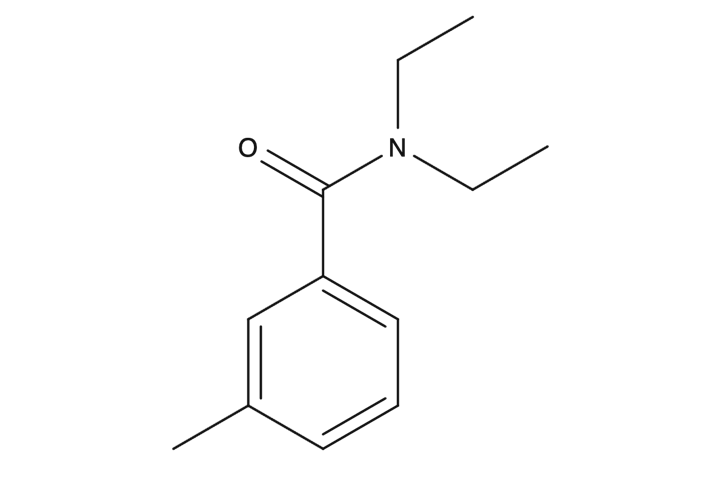
It is worth admitting that the mechanism of action of DEET for insect control is not fully understood, but there are some assumptions. Thus, one of the leading hypotheses is the assumption that DEET negatively affects the ability of insects to search for food, according to the study material. A third-party search reveals that DEET inhibits the enzymatic activity of receptors on the insects’ antennae, with the result that they cannot detect heat and carbon dioxide coming from the human body (OFF!, n.d.). In other words, the insects are confused and cannot adequately orient themselves in space. A more detailed study suggests that DEET destructively affects the limb receptors of insects, specifically mosquitoes (Dennis et al., 2019). More specifically, mosquitoes use these receptors to taste the skin, and if the skin happens to be treated with DEET, mosquitoes will not drink human blood. Notably, this is not related to bitter taste since, in the study, mosquitoes fed on the blood of people whose skin was treated with bitter-tasting substances — hence, the action of DEET is not mediated by taste characteristics.
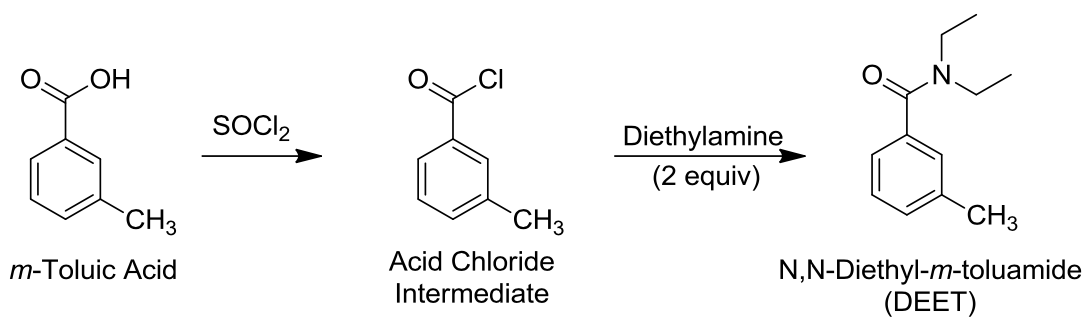
DEET is a liquid component at normal temperature so that it can be used as a liquid layer for wipes, sprays, creams, and sprays. Application of such preparation to the skin allows, as it became clear, to prevent insect bites, which in turn has a favorable effect not only on the comfort of people but also on reducing the spread of vector-borne infections. DEET was synthesized as part of the present experiment. The basic scheme of the synthesis is shown in Figure 2 — it is clearly seen that DEET is obtained by sulfochlorination (p. Reed) of toluene acid or meta-methyl benzoic acid. An amine is then added to the product, which replaces the halogen functional group with an amino group with a hydrocarbon radical.
Procedure
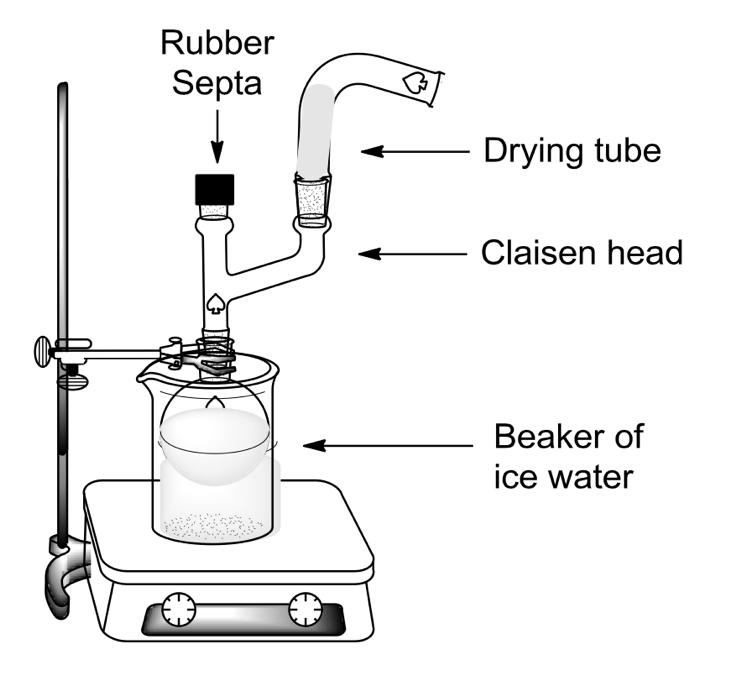
To synthesize DEET, an ice bath is used in a 1 to 1 ratio of ice to water. Toluoyl chloride of 0.5 mL (22.32 mmol) is mixed with the same amount of anhydrous ether and set up in the mechanism shown in Figure 3. To the mixture, 0.660 mL (29.46 µmol) of diethylamine is added after cooling the solution through a Claisen needle. Adding the diethylamine is similar to titrating the mixture because the addition must be done in a dropwise fashion at a rate of approximately one drop per ten seconds. The mixture is removed from the ice bath setting, after which 2 mL of 2.5M sodium hydroxide solution is added to dissolve the solid residue. Then, a hot bath is used to remove the liquid solvent from the mixture; the mixture is heated, and an additional 10 mL (446.43 mmol) of diethyl ether is added to minimize synthesis errors. The separation of the phases and washing of the organic component is facilitated by the addition of two 10 mL (446.43 mmol) portions of hydrochloric acid each. The organic layer is dried to 1 g (7.04 mmol) with anhydrous sodium sulfate, which absorbs the excess moisture. After reheating and solvent extraction, a viscous brown liquid remains, namely crude DEET.
Spectrometric methods of analysis are used for analytical verification of the purity of the obtained DEET. In particular, the organic composition of the preparation can be established by infrared spectrometry and thermometric analysis. Since DEET has been found to have a benzene ring, an amino group, and a methyl radical bonded to the aromatic ring system, the use of these methods will determine the presence (or absence) of impurities in the crude DEET. The pure drug can be used as directed to eliminate unwanted insect effects.
References
Dennis, E. J., Goldman, O. V., & Vosshall, L. B. (2019). Aedes aegypti mosquitoes use their legs to sense DEET on contact. Current Biology, 29(9), 1551-1556.
DEP. (n.d.). Education. Deet Education Program. Web.
OFF! (n.d.). Using DEET: how insect repellent works. SC Johnson. Web.